4528
How strictly are traumatic microbleeds related to the actual diffuse axonal injury?1Department of Neurosurgery, University of Pécs, Pécs, Hungary
Synopsis
This study aims to reveal the relation between traumatic microbleeds (TMBs) on susceptibility weighted imaging and diffuse axonal injury (DAI). Regional TMB and diffusion tensor imaging (DTI) data were collected from 38 traumatic brain injury patients, and 20 control subjects. Analyses included multiple linear regression among TMB parameters, clinical variables and DTI data. Only basal ganglia area TMBs were found to be significantly related to DTI alteration indicating DAI. In general, TMBs might be rather due to microvascular vulnerability than actual DAI, however, specifically basal ganglia area TMBs might be still regarded as markers of DAI.
Introduction
Diffuse axonal injury (DAI) is a substantial pathological component of traumatic brain injury (TBI), and is highly related to the clinical state and outcome 1. However, in consequence of its microscopic range, it is basically “invisible” to standard TBI imaging protocols.
Traumatic microbleeds (TMBs) on MRI, especially on susceptibility weighted imaging (SWI) are regarded as surrogate markers of diffuse axonal injury 2, 3. However, the actual relation between TMBs and DAI remained unclear, since microbleeds were mostly related to clinical parameters, -largely influenced by extracranial, non-specific factors 4.
In order to understand the relationship between TMB occurrence and DAI, TMB parameters (presence, number, localization) were related to diffusion tensor imaging (DTI) data that have been widely accepted to be able to detect white matter microstructural damage5.
Methods
Thirty-eight adults with a closed traumatic brain injury (12 mild, 4 moderate and 22 severe) fitting strict inclusion criteria who underwent SWI, high-resolution T1 (MPRAGE)- and T2 weighted imaging (3T) and routine CT were included in the study. MRI was performed in the chronic phase (2-20 months after injury). A control group of 20 age- and sex matched healthy volunteers were included.
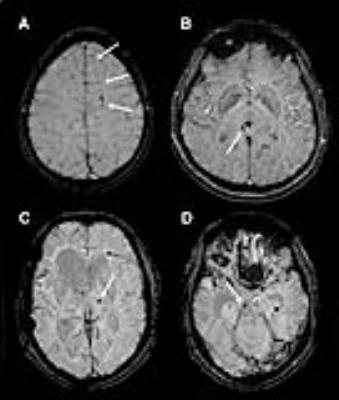
On SWI, TMB number over different regions (subcortical white matter plus centrum semiovale, corpus callosum, basal ganglia area and brainstem) were described by two raters. TMBs were defined as punctuate, ovoid or curvilinear hypointensities located in the white matter or white-grey matter border that are clearly not consistent with blood vessels or artefacts. To aid such exclusion of mimicking hypointensities and a more precise anatomical localization, both the high resolution T1 weighted- and the T2 images were registered to the SWI images using the FMRIB's Linear Image Registration Tool (FLIRT)6, which allowed a multi-modal assessment of TMB sites.
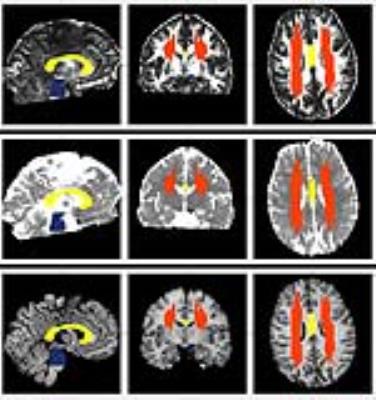
Average DTI metrics such as fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD) and radial diffusivity (RD) were also measured over the aforementioned regions using manual tracing 7 based on anatomical borders (see fig. 1). Lesion sites were excluded, DTI parameters were thus extracted from the “normal appearing white matter”. Clinical parameters including age; Glasgow Coma Scale (GCS); Rotterdam score; and TMB features (presence, regional TMB load, overall TMB load) were correlated to regional DTI parameters using multiple linear regression. TBI and control group DTI data were compared. Inter-rater reliability was tested using intraclass correlation coefficient.
Results
Most TMBs were present in the severe TBI patients, but were also found in the mild and moderate groups. The subcortical white matter and centrum semiovale was mostly affected, but the corpus callosum and basal ganglia area also showed a high number of TMBs, for TMB distribution details, see table 1, for TMB examples, see fig. 2.
In the DTI patients, FA was decreased, MD was increased over all regions, and RD was increased only in the corpus callosum significantly when compared to controls, no significant differences were found related to AD. For details, see Table 2.
Age and basal ganglia area TMB load was significantly, negatively related to the subcortical (plus centrum semiovale) white matter FA values.
GCS and age showed several significant relations with different DTI parameters over different regions.
No other significant correlations were found between clinical, imaging variables and DTI values.
For details, see Table 3.
The intraclass correlation coefficient between the two raters for TMB detections was 0.983. Discrepancies mainly arise in case of adjacent lesions, as regarding them one large or two separate smaller lesions.
Discussion
TMB distribution and the association found between GCS, age and the DTI parameters are in line with the previous studies 8-11. The DTI alterations found in the TBI patients in this study are also supported by earlier research 5, that indicates that DTI detected microstructural damage reliably over the different regions in our patients.
The novel finding of this study is that not TMBs of all regions, but only TMBs of the basal ganglia area were significantly related to any of the DTI parameters, namely the subcortical (plus centrum semiovale) white matter FA.
Conclusion
TMBs, in general seem not to be strictly related to DAI, they might be rather due to the microvascular vulnerability of the patients.
However, basal ganglia area TMBs showed a strong correlation with the microstructural damage as detected by DTI, therefore they might be regarded as DAI markers.
Based on the better understanding of the relation between TMB parameters and DAI, in order to estimate DAI in a clinical environment, a conventional lesional MRI pathology evaluation might at least in part substitute the use of quantitative DTI.
Acknowledgements
This study was funded by Hungarian Brain Research Program - Grant No. KTIA_13_NAP-A-II/8, Grant No. KTIA_13_NAP-A-II/9, SROP-4.2.2.A-11/1/KONV-2012-0017 and Hungarian Scientific Research Fund Grant No. OTKA/K-120356.References
1. Johnson, V.E., Stewart, W. and Smith, D.H. (2013). Axonal pathology in traumatic brain injury. Exp Neurol 246, 35-43.
2. Mittal, S., Wu, Z., Neelavalli, J. and Haacke, E.M. (2009). Susceptibility-weighted imaging: technical aspects and clinical applications, part 2. AJNR Am J Neuroradiol 30, 232-252.
3. Sharp, D.J. and Ham, T.E. (2011). Investigating white matter injury after mild traumatic brain injury. Curr Opin Neurol 24, 558-563.
4. Beauchamp, M.H., Beare, R., Ditchfield, M., Coleman, L., Babl, F.E., Kean, M., Crossley, L., Catroppa, C., Yeates, K.O. and Anderson, V. (2013). Susceptibility weighted imaging and its relationship to outcome after pediatric traumatic brain injury. Cortex 49, 591-598.
5. Hulkower, M.B., Poliak, D.B., Rosenbaum, S.B., Zimmerman, M.E. and Lipton, M.L. (2013). A decade of DTI in traumatic brain injury: 10 years and 100 articles later. AJNR Am J Neuroradiol 34, 2064-2074.
6. Jenkinson, M., Bannister, P., Brady, M. and Smith, S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825-841.
7. Smith, S.M., Jenkinson, M., Woolrich, M.W., Beckmann, C.F., Behrens, T.E., Johansen-Berg, H., Bannister, P.R., De Luca, M., Drobnjak, I., Flitney, D.E., Niazy, R.K., Saunders, J., Vickers, J., Zhang, Y., De Stefano, N., Brady, J.M. and Matthews, P.M. (2004). Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 Suppl 1, S208-219.
8. Ewing-Cobbs, L., Prasad, M.R., Swank, P., Kramer, L., Cox, C.S., Jr., Fletcher, J.M., Barnes, M., Zhang, X. and Hasan, K.M. (2008). Arrested development and disrupted callosal microstructure following pediatric traumatic brain injury: relation to neurobehavioral outcomes. Neuroimage 42, 1305-1315.
9. Yuan, W., Holland, S.K., Schmithorst, V.J., Walz, N.C., Cecil, K.M., Jones, B.V., Karunanayaka, P., Michaud, L. and Wade, S.L. (2007). Diffusion tensor MR imaging reveals persistent white matter alteration after traumatic brain injury experienced during early childhood. AJNR Am J Neuroradiol 28, 1919-1925.
10. Kumar, R., Husain, M., Gupta, R.K., Hasan, K.M., Haris, M., Agarwal, A.K., Pandey, C.M. and Narayana, P.A. (2009). Serial changes in the white matter diffusion tensor imaging metrics in moderate traumatic brain injury and correlation with neuro-cognitive function. J Neurotrauma 26, 481-495.
11. Di Ieva, A., Lam, T., Alcaide-Leon, P., Bharatha, A., Montanera, W. and Cusimano, M.D. (2015). Magnetic resonance susceptibility weighted imaging in neurosurgery: current applications and future perspectives. J Neurosurg, 1-13.
Figures