4527
High-Resolution DTI and Volumetric Analysis in a Mouse Model of Mild-to Moderate TBI1Radiology, Duke University Medical Center, Durham, NC, United States, 2BIAC, Duke University Medical Center, Durham, NC, 3Neurology, Duke University Medical Center, Durham, NC, 4CIVM, Duke University Medical Center, Durham
Synopsis
3D High-Resolution DTI and volumetric analyses in a mouse model of sub-acute injury after TBI with and without neuroprotective therapy.
Introduction:
Neuropathological changes following mild-to-moderate traumatic brain injury (TBI) are poorly understood1 and undetectable using conventional diagnostic imaging. Development of new imaging methods sensitive and specific to subtle injury will greatly aid clinical diagnosis and management of TBI as well as the development of new therapies. Although mouse models of TBI provide a preclinical opportunity for studying diagnostic and therapeutic strategies, the inherent challenges of small tissue volumes, motion, and acquisition time preclude systematic preclinical study of advanced MRI methods that may otherwise be readily translatable. To circumvent these challenges, high-resolution DTI and MR volumetry were performed on mouse brains ex vivo after TBI in order to characterize regional microstructural injury in the sub-acute injury phase, with and without administration of a novel neuroprotective therapeutic.Materials and Methods:
Experimental design: Total 15 mice (10-11 week old C57Cl/6J male). Normal = 3, TBI(Vehicle) = 6, TBI(treated) = 6. Treatment consisted of administration of a novel small molecule quinone oxidoreductase II (QR2) inhibitor administered i.p. 1x per week for 4 weeks, resulting in statistically significant improvement in motor function (rotorod) and cognitive performance (Morris water maze). TBI was elicited using an established closed head injury model involving a single midline impact to exposed skull with spontaneous, voluntary recovery. Mice were sacrificed and perfused 6 weeks after TBI. Following fixation, brains were removed from the skull and stored in 0.5% ProHance-doped formalin2. Image Acquisition: Mouse brains were placed in a MR compatible container filled with Fomblin and scanned on a Bruker 7T scanner using Paravision 5.1 and Quadrature volume transmit and surface receive coils. The MRI protocol comprised: (1) 3D T1-weighted FLASH sequence with FOV = 1.8 cm X 1.8 cm X 1.8 cm; matrix =256 x256 x 256; resolution = 70 x 70 x 70 μm/pixel; TE/TR = 6/30 ms; averages = 16; flip-angle = 34; scan time = 6 hrs 33 mins; (2) High resolution spin-echo based 3D DTI with FOV = 1.8 cm x 1.8 cm x 1.8cm; matrix = 128 x 128 x 128; resolution = 141 x 141 x 141 μm/pixel; TE/TR = 25/250 ms; diffusion directions = 60; Ao images = 5; B-value per direction = 1500 S/mm2 ; Scan time = 73hrs 30 mins. Post-Processing: Mouse brains were registered to a reference template from Wake forest University’s Mouse Database and segmented using ITK-SNAP. DTI parameters were calculated using TrackVis software.Results and Discussion:
Presented are a subset of analyzed
regions, specifically hippocampus (gray matter) and corpus callosum plus
external capsule (CC + EC, white matter), which demonstrate statistically
significant microstructural changes as well as treatment responses following
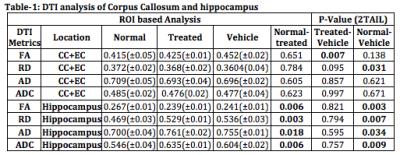
TBI (Figure-1). In CC+EC (white matter) fractional-anisotropy (FA) was increased
and radial diffusivity (RD) significantly decreased at 6 weeks following TBI,
whereas no significant change was observed for axial diffusivity (AD), fiber
density or mean diffusivity (ADC). In hippocampus (gray matter) (Figure-2) however,
FA decreased significantly and RD, AD and ADC increased (Table-1). Thus changes
in FA, RD and ADC in CC+EC (white matter) trend in opposite directions to those
in hippocampus (gray matter) in the sub-acute interval (4-6 weeks) following
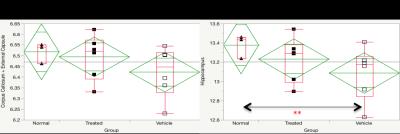
TBI. Volumetric analysis revealed a significant decrease in hippocampal but not
CC+EC volumes 6 weeks after TBI (Figure 3).
With therapy (QR2
inhibitor), DTI parameters were significantly improved in the CC+EC (white
matter) but not hippocampus, whereas volumetric changes were significantly improved
in hippocampus but not in white matter. FA and RD DTI values in CC+EC trended
towards normal whereas volume loss was mitigated in the hippocampus but not
CC+EC after therapy.
Conclusion:
Ex-vivo high resolution DTI and volumetric analyses in a mouse model of mild-to moderate TBI 6 weeks after injury reveals significant and distinct regional injury as well as response to therapy using DTI and volumetric read-outs. These clinically translatable imaging methods yield insight into regionally specific microstructural injury as well as neural targets and possible therapeutic mechanisms following mild-to-moderate TBI.Acknowledgements
No acknowledgement found.References
[1]. Bigler ED, Maxwell WL. 2012. Neuropathology of mild traumatic brain injury: relationship to neuroimaging findings. Brain Imaging Behav., Jun6(2):108-36. [2]. Johnson GA, Calabrese E, Badea A, Paxinos G, Watson C. 2012. A multidimensional magnetic resonance histology atlas of the Wistar rat brain. Neuroimage. 62:1848–1856.Figures