4526
Symptom-related Alterations of Thalamocortical Connectivity in mild Traumatic Brain Injury: An fMRI Connectome Study1Translational Imaging Research Center, College of Medicine, Taipei Medical University, Taipei, Taiwan, 2Department of Radiology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan, 3Department of Biomedical Imaging and Radiological Sciences, National Yang-Ming University, Taipei, Taiwan, 4Department of Medical Imaging, Taipei Medical University Hospital, Taipei, Taiwan, 5Department of Medical Research, Taipei Medical University Hospital, Taipei, Taiwan, 6School of Biomedical Engineering, College of Biomedical Engineering, Taipei Medical University, Taipei, Taiwan
Synopsis
Disrupted thalamocortical networks and elevated connectivity between thalamic nuclei can reveal the clinical symptoms in mild traumatic brain injury.
Background and Purpose
Mild traumatic brain injury (mTBI) is a “silent” disease commonly caused by a motor vehicle accident or contact sports injury with mild clinical symptoms. Diffuse axonal injury (DAI) is the primary neuropathology of mTBI and can be revealed by the susceptibility weighted imaging, diffusion tensor imaging, and the subsequent changes on fMRI1-4. Neuroimaging evidence that supports non-specific post-concussion symptoms after mTBI was less explored. We hypothesized that the mTBI-induced DAI may lead to the alterations of thalamocortical connectivity (also known as thalamocortical dysrhythmia5) in which the involved circuits can correlate with the post-concussion symptoms. The connectome analysis on the fMRI data was employed to identify the symptom-related changes of thalamocortical connectivity after mTBI.Materials and Methods
This study was approved by the local Institutional Review Board and the written informed consent was provided by each participant. Fifteen patients with mTBI and 17 age- and gender-matched healthy volunteers were recruited (Table 1). Inclusion criteria for patients were witnessed closed-head trauma, no focal neurologic deficit, and initial Glasgow Coma Scale higher than 13. Neuropsychological assessments were performed by a clinical psychologist to evaluate clinical symptoms. MRI data, including a 3D T1-MPRAGE (TR/TE: 2300/3.26 ms; voxel size: 1.0x1.0x1.0 mm3) and BOLD fMRI (TR/TE: 2000/20 ms; voxel size: 2.2x2.2x3.5 mm3) with 3 separated runs for resting-state, 1-back, and 2-back experiment were acquired using a 20-channel head coil on a 3T MR scanner (Siemens MAGNETOM Prisma). During n-back tasks, participants were told to respond whenever the current stimulus matched the number that had been presented n back previously (n = 1 or 2)6. An n-back session contained 3 epochs, with each composed of a 30-second task period and a 30-second fixing on a crosshair. Patients received MR scan within 2 weeks after mTBI.
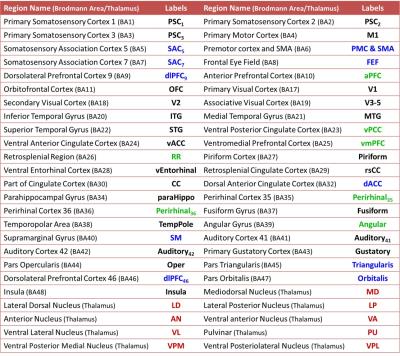
The fMRI data were preprocessed using SPM8 with the standard procedures: corrected for slice timing, realigned, spatially normalized into the standard space, and spatially smoothed with a 6-mm FWHM Gaussian kernel7. The cortical regions were parcellated based on Brodmann atlas, and the thalamic nuclei were identified based on Talairach atlas (Table 2)8. The regional BOLD signal was then extracted by averaging voxel signals within the region, and regressing out the confounding effects of motion parameters and signals from the white matter and cerebrospinal fluid. The thalamocrotical connectivity was estimated by calculating the Pearson’s correlation coefficient between regional BOLD signals (between 0.01 and 0.10 Hz) followed by Fisher’s r-to-z transform. One-sample t-test was employed to determine the significance of functional connectivity within group (p<0.05, with FDR correction), and two-sample t-test was used to determine the differences between groups (p<0.01). Partial correlation coefficients with controlling age and gender effects were computed to reveal the relations between functional connectivity and neuropsychological scores.
Results and Discussion
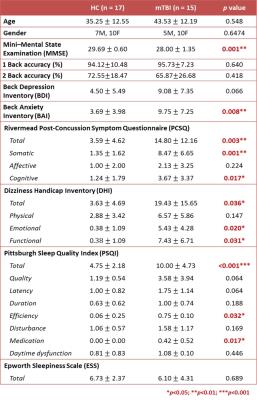
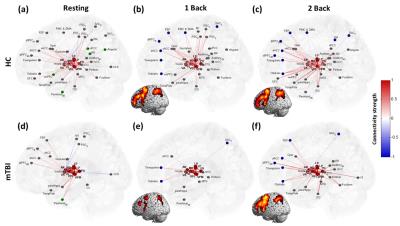
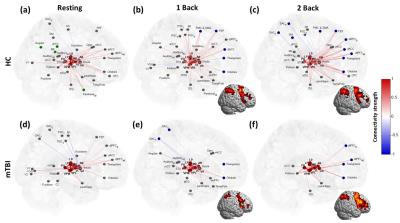
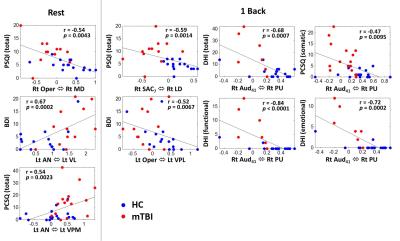
The mTBI patients showed a mild decline of cognitive performance (MMSE), an increment of anxiety (BAI), significant post-concussion symptoms (PCSQ), an evident dizziness (DHI), and worse sleep quality (PSQI) compared to healthy controls (Table 1). The connectome analysis also revealed abnormalities in mTBI patients. During the resting state, extensive connections to different cortical regions from specific thalamic nuclei were identified in healthy controls for both hemispheres (Fig.1a and Fig.2a), however the abundance of thalamocortical connectivity significantly decreased in mTBI patients (Fig.1d and Fig.2d). When performing 1-back task, increased number and strength of functional connectivity were observed in the working memory circuitry (blue nodes in Fig.1b and Fig.2b). When the work load increased to 2-back, the reorganization of thalamocortical connections became even more explicit, i.e., reallocating most of the connectivity into bilateral frontal and parietal regions (Fig.1c and Fig.2c). Although functional reorganizations were also found in patients, several dorsolateral prefrontal, premotor, and posterior parietal regions were absent during the n-back tasks, especially the 1-back condition (Fig.1e, 1f, 2e, and 2f). Interestingly, the patterns of thalamocortical connections matched the cortical activations during n-back tasks (as shown in the lower left/right corner of subplots in Fig.1 and Fig.2), suggesting that the network reorganization modulated by thalamic nuclei may be critical in working memory. Finally, the correlation analysis revealed that the elevated connectivity between thalamic nuclei, one of the characteristics of thalamocortical dysrhythmia5, can be positively correlated to depression and post-concussion symptoms (Fig.3b and 3c). Disruptions of thalamocortical connectivity in several other regions also significantly correlated to the PSQI, BDI, and DHI scores (Fig. 3).Conclusions
This study reported the alterations of thalamocortical connectivity in patients with mTBI and the corresponding relations with the clinical symptoms. A continuous study on a larger study population is warranted to identify reliable image biomarkers for assisting clinical diagnosis and treatment in mTBI patients.Acknowledgements
This study was funded in part by the Taipei Medical University (TMU103-AE1-B20) and the Ministry of Science and Technology (MOST 105-2314-B-038-014, MOST 104-2923-B-038 -003 -MY3), Taipei, Taiwan.References
1. Bigler ED, Maxwell WL. Neuropathology of mild traumatic brain injury: relationship to neuroimaging findings. Brain imaging and behavior. 2012;6(2):108-36.
2. Huang YL, Kuo YS, Tseng YC, Chen DY, Chiu WT, Chen CJ. Susceptibility-weighted MRI in mild traumatic brain injury. Neurology. 2015;84(6):580-5.
3. Inglese M, Makani S, Johnson G, Cohen BA, Silver JA, Gonen O, Grossman RI. Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. Journal of neurosurgery. 2005;103(2):298-303.
4. Chen CJ, Wu CH, Liao YP, Hsu HL, Tseng YC, Liu HL, Chiu WT. Working memory in patients with mild traumatic brain injury: functional MR imaging analysis. Radiology. 2012;264(3):844-51.
5. Llinás R, Ribary U, Jeanmonod D, Cancro R, Kronberg E, Schulman J, Zonenshayn M, Magnin M, Morel A, Siegmund M. Thalamocortical dysrhythmia I. Functional and imaging aspects. Thalamus & Related Systems. 2001;1(03):237-44.
6. Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Human brain mapping. 2005;25(1):46-59.
7. Penny WD, Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE, editors. Statistical parametric mapping: the analysis of functional brain images. Academic press; 2011 Apr 28.
8. Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Human brain mapping. 2000 Jul 1;10(3):120-31.
9. Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. Journal of cognitive neuroscience. 2009;21(3):489-510.
10. Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Human brain mapping. 2005;25(1):46-59.
Figures