4521
Detecting perfusion deficits in concussive blast subjects using arterial spin labelingSwati Rane1, Jalal B Andre2, and Christine MacDonald3
1Radiology, University of Washington Medical Center, Seattle, WA, United States, 2Neuroradiology, University of Washington Medical Center, Seattle, WA, United States, 3Neurological Surgery, University of Washington Medical Center, Seattle, WA, United States
Synopsis
This work applied ASL imaging to understand perfusion abnormalities in concussive brain injury. Results show overall reduction in cerebral perfusion, with significant decreases in the frontal and temporal lobes as well as the insula.
PURPOSE
Traumatic brain injury (TBI) is a heterogeneous injury that can cause a multitude of pathoanatomic brain changes. Mild or concussive TBI is often difficult to detect given the more subtle nature of insult1. Advanced neuroimaging approaches have the potential to elucidate these underlying brain changes currently not appreciated on standard structural acquisitions. The purpose of the current study was to apply arterial spin labeling (ASL) to assess possible perfusion deficits in individuals with mild blast-related TBI using pseudo-continuous ASL imaging2. Subjects were drawn from the Assessment of long term outcome & Disability in Active-duty military Prospectively examined following concussive TBI (ADAPT) study, which prospectively follows active-duty US military after a concussive injury and matched combat-deployed controls with advanced MRI and clinical evaluation.METHODS
Experiment: The study involved two groups of subjects; those with a concussive brain injury from blast during deployment, and those without history of blast exposure and no diagnosis of brain injury from deployment i.e., controls. MRI examination included: sequential 3D T1-weighted images for image registration purposes, phase contrast angiography obtained for optimal ASL label slab placement (perpendicular to all ascending arteries at approximately 90 mm from the AC-PC line), and a pseudo-continuous ASL (pCASL) acquisition using body coil transmission and SENSE 32-channel reception (3T; Philips Achieva). The pCASL parameters were: matrix = 96×96×20, spatial resolution = 3×3×5mm3, flip angle = 90°, TE = 19 ms, TR = 5000 ms, label duration = 1800 ms, post-labeling delay = 2000 ms, 30 control/label pairs and a M0 image with TR = 10000 ms, R = 2.5. Analysis: Images were first motion-corrected and registered to the M0 image. Pairwise subtraction of the control and label pairs was performed and CBF was calculated based on the ISMRM’s recommendations for quantification of ASL data. The CBF maps were registered to a standard 2mm MNI template using FSL-FLIRT using intra-modal registration with search angles restricted to -30 and 30 in the X,Y, and Z directions. Accuracy of registrations was visually verified. For subjects with imperfect registrations, the search angles were altered to improve congruence with the MNI template. Two control subjects failed image registration and were excluded. CBF was calculated in the frontal, parietal, temporal, insular, and occipital cortices using the MNI atlas in FSL. Measurements were also made in the Caudate, Putamen, Thalamus, Hippocampus, and Amygdala using the Harvard-Oxford atlas in FSL. CBF values were compared between the two groups using a 1-sided t-test and corrected for multiple comparisons using Bonferroni adjustment (p-value for significance = 0.05/10 = 0.005).RESULTS
44 participants underwent pCASL imaging following informed, written consent (gender = 5F/31M, n = 36 controls, age = 34±8 years, years of education = 15±2 years, 8 TBI, age=32±8 years, years of education = 13±1 years). Since all TBI subjects were males, only the 31 male controls were included in this sub-analysis. Figure 1 depicts the perfusion map for a representative control and TBI subject. Overall reduced perfusion is evident in the TBI subject compared to the normal control. All brain regions showed reduced perfusion in the TBI group compared to the control subjects (Figure 2). After correction for multiple comparisons, CBF was significantly reduced in the frontal lobe, insula, and the temporal lobe (p<0.005) in the TBI group (CBF in frontal, insular, and temporal lobes 20±4, 22±5, 24±4 ml/100g/min respectively) compared to the controls (CBF in frontal, insular, and temporal lobes 26±4, 28±6, 29±5 ml/100g/min respectively).DISCUSSION
Our study showed decreased overall perfusion in mild TBI subjects compared to controls subjects with significant reductions in the frontal and temporal lobes as well as the insula. While most studies of TBI focus on diffuse axonal injury in the white matter, very few studies evaluate perfusion deficits in TBI. Our results are in accordance with these studies reporting decreased perfusion in similar brain regions3-5. Perfusion imaging can be combined with imaging measures of anatomical disruptions to better characterize the pathological sequelae of TBI and potentially subsequent impairment. TBI subjects in this study are young adults and are less likely to have comorbid cerebrovascular disease. Therefore, the observed differences are likely to be an effect of the head injury exposure. However, concussive injuries may have varying points of impact and the above results likely reflect common brain regions that are most susceptible to trauma. Future work will involve assessing subjects on an individual basis to assess subject-specific pattern of perfusion abnormalities that is related to the location and degree of impact.CONCLUSION
We have shown the applicability of ASL to identify perfusion deficits in mild TBI subjects.Acknowledgements
No acknowledgement found.References
1. Lee B, Newberg A. Neuroimaging in traumatic brain imaging. NeuroRx. 2005 Apr 30;2(2):372-83. 2. Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L, Lu H, MacIntosh BJ, Parkes LM, Smits M, Osch MJ. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magnetic resonance in medicine. 2015 Jan 1;73(1):102-16. 3. Wang Y, West JD, Bailey JN, Westfall DR, Xiao H, Arnold TW, Kersey PA, Saykin AJ, McDonald BC. Decreased cerebral blood flow in chronic pediatric mild TBI: an MRI perfusion study. Developmental neuropsychology. 2015 Jan 2;40(1):40-4. 4. Amen DG, Willeumier K, Omalu B, Newberg A, Raghavendra C, Raji CA. Perfusion Neuroimaging Abnormalities Alone Distinguish National Football League Players from a Healthy Population. Journal of Alzheimer's Disease. 2016 Apr 25(Preprint):1-5. 5. Barlow K, Lorenzo M, Calson H, Marc L. Cerebral Perfusion Changes in Children with and without Post-Concussion Syndrome Following a Mild Traumatic Brain Injury Using Arterial Spin Labelling (P3. 321). Neurology. 2016 Apr 5;86(16 Supplement):P3-321.Figures
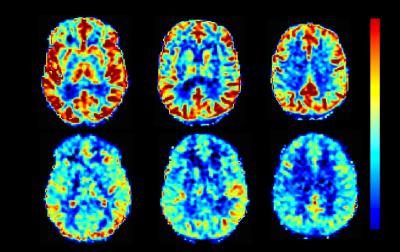
Figure 1: Perfusion map for a representative control
and TBI subject. Overall reduced perfusion is evident in the TBI subject (mean
age: 35 years) compared to the controls subject (mean age: 38 years), matched
for 14 years of education
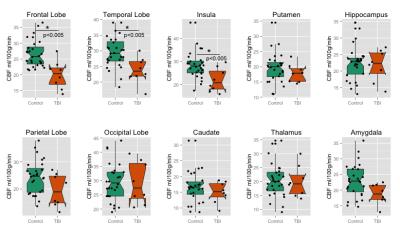
Figure 2: Region-wise comparison
of CBF in normal controls and TBI subjects. Significant difference after
correction for multiple comparisons were observed in the frontal lobe, temporal
lobe and insular cortex.