4520
Early NAA Reductions predict Neuropsychological Outcomes after Pediatric TBI1Radiology, Loma Linda University, Loma Linda, CA, United States, 2Pediatric Psychology, Loma Linda University, Loma Linda, CA, United States, 3Pediatric Neurology, Loma Linda University, Loma Linda, CA, United States
Synopsis
The primary aim of this prospective study was to test the hypothesis that early 3D MR spectroscopic (MRSI) changes in discrete regions of the brain after TBI predict neuropsychologic outcomes 1 year after injury. MRSI was acquired at 3T in 68 pediatric mild to severe TBI subjects and 72 controls. Subacute NAA/Cr and NAA/Cho ratios were significantly reduced in TBI patients in all brain regions. A binary logistic regression analysis using combined subcortical NAA/Cr ratios alone predicted dichotomized neurologic outcome (93%), Full Scale IQ (78%), General Memory (82%) and General Attention (88%).
Body of Abstract
Introduction: Often after traumatic brain injury (TBI), there is a discrepancy between functional deficits and imaging findings. It is well known that TBI is a diffuse injury that can produce a cascade of cellular and metabolic alterations1. The severity and location of injury greatly affects the degree of alterations. A technique that can simultaneously sample multiple regions of the brain appears to be necessary for fully evaluating the heterogeneity and distribution of injury. Whether the injury is mild or severe, tools are needed to reliably predict patient outcome that can be used to direct clinical decisions and long term rehabilitation efforts. Proton MR Spectroscopy (MRS), studies have demonstrated that changes in key brain metabolites such as N-acetylaspartate (NAA), a measure of neuronal loss or dysfunction and choline (Cho) a measure of membrane synthesis or disruption can detect injury in brain that may be remote from TBI lesions and appear normal on structural MRI2,3. We present our findings on a prospective study of 3D proton magnetic resonance spectroscopic imaging (MRSI) in pediatric patients with mild to severe injury, acutely and at 1-year after injury. The primary aim of this study was to test the hypothesis that early MRS changes in discrete regions of the brain after TBI predict neuropsychologic outcomes 1 year after injury.
Methods: Enrollment: In this prospective IRB approved study, children 4-18 years old with TBI were included if initial GCS was between 3-12 and GCS 13-15 if the first imaging study was positive for hemorrhage/contusion. Controls with no history of head injury were included. Imaging: Using a 3T system (Trio/Tim, Siemens Medical Solutions, Erlangen, Germany) imaging included 3D-T1W MPRAGE, 3D-T2W SPACE, FLAIR, Susceptibility Weighted Imaging (SWI) and Diffusion Tensor Imaging (DTI). Spectroscopy: 3D proton MR spectroscopic imaging (MRSI) was acquired with TR/TE=1700/144 msec in four or five 10 mm thick slabs covering the level of the corpus callosum through the brain stem. LCModel (Stephen Provencher Inc., Oakville, Ontario, Canada; Version 6.0) was used for post processing to obtain mean N-acetyl-aspartate (NAA)/creatine(Cr), NAA/choline(Cho) and Cho/Cr ratios for each region.T1W images were used for brain segmentation (SPM 7). Each voxel was assigned to one of 12 anatomic brain regions and 3 combined subregions as follows: Superficial Brain (SB)= Occipital Gray Matter (OG), Parietal White (PW) and Gray Matter (PG), Temporal White (TW) and Gray (TG) Matter, Frontal White (FW) and Gray Matter (FG); Subcortical Brain (SC) = Basal Ganglia (BG), Thalamus (TH), and corpus callosum (CC); Posterior Fossa (PF) = Brain Stem (BS) and Cerebellum (CB). Timing: TBI patients were scanned within 6-17 days and 1 year after injury. Controls were scanned twice, 1 year apart. A neuropsychologic evaluation was done at 3 months and at 1 year after injury for patients and initially and at 1 year for controls to evaluate the following: FSIQ: Wechsler Abbreviated Scale of Intelligence (WASI Full Scale) and subtest PIQ: Visual Spatial Processing, Memory: Children’s Memory Scale (CMS), Attention: Test of Everyday Attention- Children (TEA-CH G score). A neurologic evaluation was done at 3, 6 and 12 months for patients and initially and at 1 year for controls. A Pediatric Cerebral Performance Category Scale score (PCPCS) was assigned by a pediatric neurologist to assess neurologic outcome. Regional MRS ratios for the initial studies were correlated and regression analyses were done to determine which variables predicted neurologic (PCPCS) and neuropsychological outcomes at 12 months.
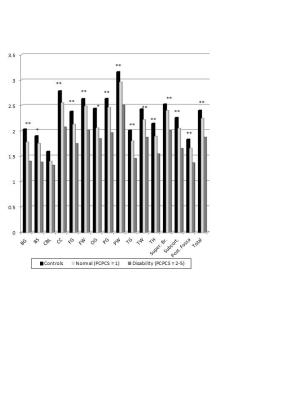
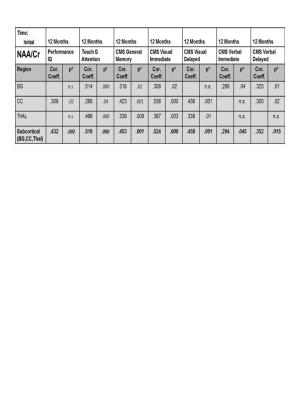
Results: We studied 68 children (50M/18F); mean age was 11.9 ±3.6 yrs (4.4-18.0yrs); initial GCS (Mild=25; Moderate=9; Severe=34) and 72 control children; mean age 12.7±3.3 yrs (5.4-18.4 yrs). Initial studies were done at 11.5±3.5 days after injury and follow-up studies were done at 12.2±0.8 months for TBI patients and 12.8±1.0 months for controls. 3D MRSI showed significant reductions in NAA/Cr and NAA/Cho ratios in TBI patients on the initial study performed at the subacute stage (7-17 days after injury) in all brain regions sampled (Figure 1). Reduction of NAA in the subcortical region, consisting of the BG, CC, and TH, showed the strongest, most significant correlations to visual spatial processing (PIQ), Attention, General Memory and Immediate and Delayed Visual tests and with lesser significance for Immediate and Delayed Verbal tests (Figure 2). A binary logistic regression analysis using combined subcortical NAA/Cr ratios alone predicted dichotomized PCPCS (93%), FSIQ (78%), General Memory (82%) and General Attention (88%) (p≤.01).
Conclusions: At the subacute stage, reduction of NAA caused by neuronal loss or dysfunction is a sensitive marker of injury that can be used to predict long-term (12 month) neurologic, and neuropsychologic outcomes.
Acknowledgements
This project was supported by Award Number 5R01NS054001 from the National Institute of Neurological Disorders and Stroke. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the NIH.References
1. Farkas O, Povlishock JT. Cellular and subcellular change evoked by diffuse traumatic brain injury: a complex web of change extending far beyond focal damage. Prog Brain Res 2007;161:43-59.
2. Govind V, Gold S, Kaliannan K, et al. Whole-brain proton MR spectroscopic imaging of mild-to-moderate traumatic brain injury and correlation with neuropsychological deficits. J Neurotrauma. 2010;27:483-496.
3. Gasparovic C, Yeo R, Manell M, et al. Neurometabolite concentrations in gray and white matter in mild traumatic brain injury: an 1H-magnetic resonance spectroscopy study. J Neurotrauma. 2009;26:1635-1643.
Figures