4518
Evolving Functional Connectivity in Rats following Mild Traumatic Brain Injury1Translational Imaging Research Center, College of Medicine, Taipei Medical University, Taipei, Taiwan, 2Department of Radiology, School of Medicine, Taipei Medical University, Taipei, Taiwan, 3Department of Biomedical Imaging and Radiological Sciences, National Yang-Ming University, Taipei, Taiwan, 4Department of Medical Research, Taipei Medical University Hospital, Taipei, Taiwan, 5Department of Medical Imaging, Taipei Medical University Hospital, Taipei, Taiwan, 6School of Biomedical Engineering, College of Biomedical Engineering, Taipei Medical University, Taipei, Taiwan, 7Department of Biomedica, China Medical University, Taichung, Taiwan
Synopsis
Longitudinal rsfMRI showed the hyper-connectivity in the primary somatosensory cortex and DMN in the acute phase after experimental impact acceleration injury. This is the first demonstration of functional connectivity change with the preserved brain structure after mTBI in rats.
Introduction
While no significant structural change in the early phase after Mild traumatic brain injury (mTBI), patients may claim a variety of post-concussive symptoms (PSC) after weeks of the injury, which affect the quality of their life.1 The negative finding in routine neuroimaging, namely CT and MRI, immediately after mTBI hinders the early diagnosis and prediction of long-term PSC.2 To discover early biomarkers for mTBI, some clinical studies have highlighted the consequences of mTBI on functional connectivity using resting-state functional MRI (rsfMRI).3 However, the evolution of neuronal network after mTBI has not been well explored due to extensive causes of mTBI, different injury types, indefinable trauma location, as well as the limited acute mTBI patients in clinical practice. Herein, we aim to monitor dynamics of functional connectivity by using rsfMRI in rats following modified impact acceleration injury (IAI), a closed-head and helmet-worn mTBI model.4 Longitudinal rsfMRI was performed up to 50 days after injury. A significant increase of regional strength and/or connectivity was observed in the primary sensory cortex and default mode network (DMN) at 24 h after IAI. Our finding shows that the alteration of brain connectivity prior to detectable structural damage in experimental mTBI model for the first time, which may shed light on early diagnosis of mTBI.Methods
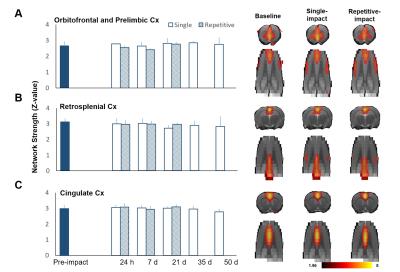
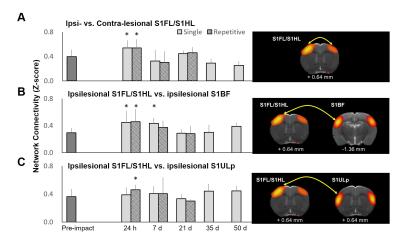
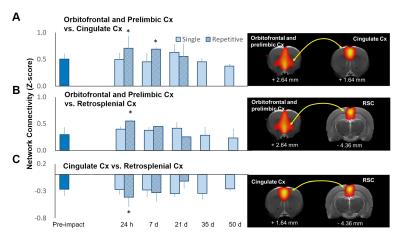
Male Sprague–Dawley rats (weighing 250–400 g) were anesthetized with Chloral Hydrate and placed in a stereotactic frame for IAI. A weight of 600 g was dropped from a height of 1 m through a stainless-steel tube on the secured impact aiming at the metal helmet cemented on the skull of the forelimb region of primary sensory cortex (S1FL, 1.5 mm posterior and 2.5 mm lateral to bregma). Animals were injured with single (n=6) or repetitive IAI within 1 h (n=6). Longitudinal MRI was performed pre, at 24 h, day 7, 21, 35 and 50 after IAI. MRI in rats was performed on a Bruker 7 T PharmaScan with a volume coil as the transmitter and a rat surface coil as the receiver. After the bolus injection of dexmedetomidine (0.015 mg/kg), continuous infusion of dexmedetomidine (0.03 mg/kg/h) was performed with low-dose isoflurane (0.25%) during the whole MR protocol.5 rsfMRI was performed after 90 min over infusion and acquired using the single-shot echo-planar imaging (EPI) with TR/TE= 1000/15 ms, FOV= 3.5 ×3.5 cm, matrix size = 64×64, 16 slices, slice thickness of 1 mm and number of repetition= 300 for total scan time= 5 min. T2-weighted images with the same geometry were also acquired. Slice timing and spatial smoothing were performed on SPM8 after pre-processing. rsfMRI data were analyzed using gICA with 30 components by GIFT software package. Statistical analysis between different time points was conducted using dual-regression approach.6 Pearson correlation coefficient was calculated and presented after Z transformation. Major functional connectivity map of our interest included 1) the barrel field region (S1BF), the forelimb/hindlimb region (S1FL/S1HL) and the upper lip region (S1ULp) of the primary sensory cortex, correlated to the impact target, and 2) the default mode network (DMN) in rats encompassing the orbitofrontal and prelimbic cortex, the cingulate cortex, and the retrosplenial cortex.5Results & Discussion
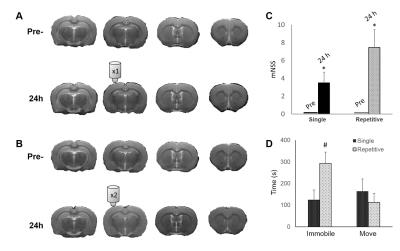
Animals showed no significant brain lesion in T2-weighted images at 24 h after IAI (Fig. 1A&B), while the modified neurological severity (mNSS) score raised (4 and 8 in single and repetitive injury, respectively) after injury (Fig. 1C), suggesting that the experimental mTBI with significant behavioral deficit but without tissue damage in the brain was used. Interhemispheric functional connectivity within S1FL/S1HL, as well as S1BF, was enhanced at 24 h after single and repetitive IAI (Fig 2A&B). Functional connectivity was increased between ipsi- and contra-lesional S1FL/S1HL (Fig 4A) and among cortical regions in the ipsi-lesional primary somatosensory cortex (Fig 4B&C) at 24 h after IAI. Taken together, acute neural functional compensation was found in hyper-connectivity to contra-lesional sensory region and wider cortical recruitment in the ipsi-lesional regions. The default mode network (DMN) in rats was clearly identified in our animals, suggesting the animal condition was well preserved. While no differences in within-connectivity strength among DMN was observed (Fig 3), functional connectivity between regions among DMN was significantly enhanced at 24 h after repetitive IAI (Fig 5). The hyperconnectivity among DMN may be an index of stress-induced symptom, which also correlates to the immobility of rats after repetitive IAI (Fig. 1D).7 This is the first demonstration of longitudinal functional connectivity change in the preserved brain structure of mTBI in rats. Further studies will attempt to explore the functional connectivity and its behavioral outcome correlates.Acknowledgements
This study was funded in part by the Taipei Medical University (TMU103-AE1-B27) and the Ministry of Science and Technology (MOST 105-2628-B-038-002-MY2 and MOST 104-2923-B-038-003-MY3), Taipei, Taiwan.References
1. Gosselin N and Tellier M, Patients with traumatic brain injury are at high risk of developing chronic sleep-wake disturbances. J Neurol Neurosurg Psychiatry, 2010. 81(12): 1297.
2. Shenton M E, Hamoda H M, Schneiderman J S, et al., A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav, 2012. 6(2): 137-92.
3. Zhou Y, Milham M P, Lui Y W, et al., Default-mode network disruption in mild traumatic brain injury. Radiology, 2012. 265(3): 882-92.
4. Fujita M, Oda Y, Wei E P, et al., The combination of either tempol or FK506 with delayed hypothermia: implications for traumatically induced microvascular and axonal protection. J Neurotrauma, 2011. 28(7): 1209-18.
5. Lu H, Zou Q, Gu H, et al., Rat brains also have a default mode network. Proc Natl Acad Sci U S A, 2012. 109(10): 3979-84.
6. Filippini N, MacIntosh B J, Hough M G, et al., Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A, 2009. 106(17): 7209-14.
7. Henckens M J, van der Marel K, van der Toorn A, et al., Stress-induced alterations in large-scale functional networks of the rodent brain. Neuroimage, 2015. 105: 312-22.
Figures