4514
Therapeutic Effect of Bone Marrow Stromal Cells on Vascular Permeability and Hemodynamic Alteration in Traumatic Injured Brain: A long-term MRI Study1Neurology, Henry Ford Health System, Detroit, MI, United States, 2Physics, Oakland University, Rochester, MI, United States, 3Neurosurgery, Henry Ford Health System, Detroit, MI, United States
Synopsis
Cerebral
vascular permeability and hemodynamic alteration in a broad normal appearing
brain tissue in response to the transplantation of hMSCs after TBI were longitudinally
investigated up to 3-months post-injury. Our data reveal the evidence that a quicker recovery
of vascular integrity, as a result of cell transplantation, is associated with a higher
level of cerebral perfusion, and acute cell administration after TBI
significantly promotes these global therapeutic effects. The findings of the current study indicate that BBB reconstitution plays an essential role in CBF restoration in the injured
brain, which in turn, contributes to the improvement of functional outcome.
Background and Purpose
Pathophysiological consequences of traumatic brain injury (TBI) that are crucially involved in secondary brain damage, blood-brain barrier (BBB) breakdown and hemodynamic disruption after TBI have attracted a great deal of attention as important targets for therapeutic intervention.1 Cell transplantation following TBI facilitates BBB reconstitution and perfusion recovery.2, 3 However, there are few studies documenting the dynamic relationship between the degree of BBB damage and perfusion status post-injury, especially in a broad region of normal appearing cerebral tissue, and how the cell therapy affects such evolution patterns. To address these issues, the current study was undertaken to investigate the long-term and global response of cerebral vascular permeability and hemodynamic alteration in traumatic injured brain to the engraftment of human bone marrow stromal cells (hMSCs).Materials and Methods
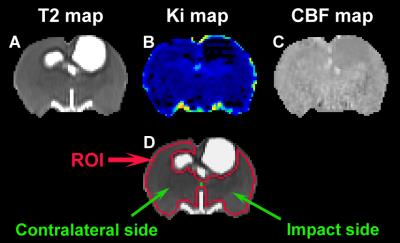
Male Wistar rats (300-350g, n=30) subjected to controlled cortical impact TBI were intravenously injected with 1 ml of saline (n=5/group, at 6 hours or 1 week post-injury) or hMSCs in suspension (~3x106 hMSCs, n=10/group, at 6 hours or 1 week post-injury). In vivo MRI acquisitions (T2-weighted imaging, cerebral blood flow (CBF) and blood-to-brain transfer constant (Ki) of Gd-DTPA) and neurological behavioral estimates (water maze test) were performed on all animals at multiple time points up to 3 months post-injury. Values of Ki and CBF were dynamically monitored in regions encompassing the ipsilateral or contralateral hemisphere as well as in both hemispheres of the brain (Fig 1, ROI).Results
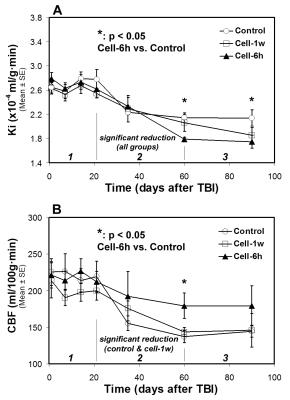
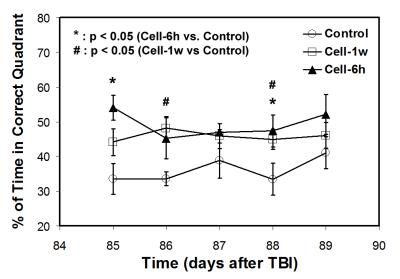
All animals with saline injection post-TBI were considered as a saline-treated group since no significant differences in MRI measurements and functional outcomes between the acute (6 hours) and delayed (1 week) saline-treated group were detected. Our long-term imaging data show that BBB breakdown and hemodynamic disruption result from an unilateral focal injury, as revealed by Ki and CBF, respectively, and affect both hemispheres of the brain. However, only within an early short period (less than 1 week post-TBI), significantly increased abnormal values of Ki and CBF were present in the ipsilateral side compared to in the contralateral side of the injured brain. Temporal profiles of Ki (Fig. 2A) and CBF (Fig. 2B) for all treatment groups exhibit a general three-stage evolution pattern, with TBI-induced initial disturbance of Ki and CBF lasting for about 3-weeks (stage 1), followed by a dramatic change in Ki and CBF between 3-weeks and 2-months (stage 2) and a reduced variation of these parameters after 2-months (stage 3). Cell engraftment (acute or delayed) after TBI mainly alters Ki and CBF profiles in stage 2 and leads to lower Ki and higher CBF values in stage 3 compared to saline administration (Fig. 2). Significantly improved neurological performance, as measured by water maze test, was detected in the cell-treated groups compared to the saline-treated group (Fig. 3).Discussion and Conclusions
We found a three-stage temporal profile of both Ki and CBF post-TBI, and the benefits of administration of hMSCs, e.g., decreasing Ki and increasing CBF, started from stage 2 and continued to stage 3. Our data reveal the evidence that a quicker recovery of vascular integrity, as a result of cell transplantation, is associated with a higher level of cerebral perfusion, and acute cell administration after TBI significantly promotes these global therapeutic effects. The findings of the current study indicate that BBB reconstitution plays an essential role in CBF restoration in the injured brain, which in turn, contributes to the improvement of functional outcome.Acknowledgements
This work was supported by National Institutes of Health RO1 NS064134.References
1. Shlosberg D, Benifla M, Kaufer D, et al. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nature reviews. Neurology. 2010;6:393-403 2.
2. Menge T, Zhao Y, Zhao J, et al. Mesenchymal stem cells regulate blood-brain barrier integrity through timp3 release after traumatic brain injury. Science translational medicine. 2012;4:161ra150 3.
3. Li L, Jiang Q, Qu CS, et al. Transplantation of marrow stromal cells restores cerebral blood flow and reduces cerebral atrophy in rats with traumatic brain injury: In vivo mri study. Journal of neurotrauma. 2011;28:535-545
Figures