4513
Decreased apparent fibre density in an experimental model of traumatic brain injury1Department of Anatomy and Neuroscience, The University of Melbourne, Melbourne, Australia, 2The Florey Institute of Neuroscience and Mental Health, Parkville, Australia, 3Department of Electrical & Electronic Engineering, The University of Melbourne, Australia, 4National Institute of Radiological Sciences, Japan, 5Department of Anatomy and Neuroscience, The University of Melbourne, Australia, 6Department of Medicine, The University of Melbourne, Australia
Synopsis
Apparent fibre density (AFD) is postulated to be a sensitive marker of white matter damage and, as it is derived from a continuous fibre orientation distribution, may identify changes along single fibre bundles in regions containing multiple fibre groups. Here, we compared AFD to traditional DTI metrics in an experimental model of traumatic brain injury (TBI). We found that rats given a TBI had widespread regions of reduced AFD when compared to sham-injured rats as well as significant, but less extensive changes in DTI metrics. These results support the use of AFD in assessing disease progression and treatment following TBI.
Introduction
Traumatic brain injury (TBI) has typically been assessed with diffusion tensor imaging (DTI), an MRI method commonly used as a marker for white matter integrity. However, as the DTI model fits a single fibre orientation, results can become confounded in regions of ‘crossing’ white matter fibres. In contrast, constrained spherical deconvolution (CSD) estimates a continuous fibre orientation distribution (FOD) directly from high angular resolution diffusion weighted images (HARDI). Apparent fibre density (AFD) is derived from the FOD and is postulated to be a sensitive marker of white matter damage.2 Additionally, as it is derived from the FOD, AFD may be able to identify changes along single fibre bundles in regions containing multiple fibre groups and therefore may be more sensitive to white matter injury following TBI. Here we investigated changes in AFD following experimental TBI and compared these with traditional DTI metrics.
Methods
Twenty male Long-Evans hooded rats underwent either a lateral fluid percussion injury (FPI, n = 11 of which 8 survived) or sham injury (n = 9). Sham injured rats had a craniotomy but didn't receive a fluid percussion pulse. A HARDI dataset was acquired at 12 weeks post-injury using a 4.7 Tesla Bruker Avance III scanner fitted with a BGA12S2 gradient set and actively decoupled volume transmit and 4-channel surface receive coils. Imaging parameters included: TR = 9 s, TE= 37 ms, FOV = 38.4 × 38.4 mm2, partial parallel imaging acceleration = 2, matrix size = 128 × 128, slices = 36 and isotropic spatial resolution = 3003 μm3. Diffusion weighting was performed in 126 directions with δ = 5 ms, Δ = 14 ms and b-value = 3000 s/mm2. Ten non-diffusion images (b0) were also acquired. AFD computation and analysis was performed as outlined previously.2 HARDI datasets were corrected for spatial intensity inhomogeneity and normalized to the cerebrospinal fluid b0 signal intensity. After upsampling by a factor of two, FODs were computed by CSD using the MRtrix software package (www.mrtrix.org) with a group average response function. For voxel-wise analysis of AFD, a study-specific template was generated using symmetric diffeomorphic FOD registration with sham-injured rat FODs.3 FOD images for all rats were then registered to the study-specific template, and modulated to account for changes in bundle width due to atrophy or hypertrophy. AFD images were computed for all registered FODs and group differences were assessed using connectivity-based fixel enhancement (CFE).4 CFE uses probabilistic tractography to identify voxels that are connected by specific fibre populations and therefore are more likely to share underlying pathology. Here, we generated a whole-brain tractogram with 20 million streamlines using the iFOD2 tractography algorithm and the following parameters: angle = 22.5°, max length = 25 mm, min length = 0.5 mm and power = 1. To reduce tractography reconstruction biases we applied the spherical deconvolution informed filtering of tractograms (SIFT) method,5 terminating with a final count of two million streamlines. Statistical inference was performed using a general linear model (GLM) and non-parametric permutation testing with 5,000 permutations, fully corrected for family-wise error (FWE). A p-value < 0.005 was considered significant. For comparison we also performed a whole-brain voxel-wise analysis of DTI metrics using non-parametric permutation testing to compare the various DTI metrics across the whole brain. DTI metrics were reconstructed and transformed into the study-specific template space using MRtrix. Voxelwise cross-subject statistical analysis was performed with 5,000 permutations and a FWE-corrected p-value < 0.005 was considered significant.Results
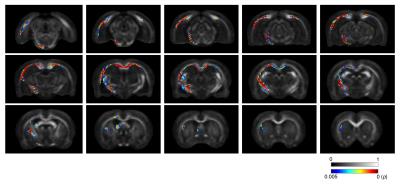
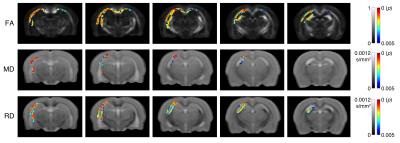
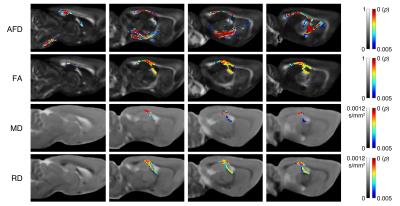
As shown in Figure 1, a significant decrease in AFD was observed in TBI rats compared to sham-injured rats. Regions affected were predominantly ipsilateral to the injury and included the corticospinal tract, external capsule, fornix, and the corpus callosum. Reduced AFD extended along the corpus callosum from the ipsilateral to the contralateral hemisphere. In comparison, although analysis of whole-brain DTI metrics revealed widespread regions of significantly decreased FA and increased MD and RD following TBI (Fig 2), the DTI metrics appeared to be less sensitive than AFD (Fig 3).Discussion
Regardless of the method used, the observed diffusion changes in this study were predominantly found within the corpus callosum, external capsule, and fimbria, all of which are white matter bundles commonly damaged following FPI in the rat. Notably, CFE-based analysis of AFD was the only method that identified damage within the corticospinal tract of rats given an FPI, which emphasizes the potential sensitivity of this method. Although future studies are required to investigate the specificity of AFD over DTI metrics, our findings here suggest that AFD may improve our understanding of disease progression and treatment following TBI.Acknowledgements
No acknowledgement found.References
1. Tournier J-D, Calamante F, Gadian D, et al. Direct estimation of the fiber orientation density function from diffusion-weighted MRI data using spherical deconvolution. Neuroimage 2004;23:1176–1185.
2. Raffelt D, Tournier J-D, Rose S, et al. Apparent Fibre Density: a novel measure for the analysis of diffusion-weighted magnetic resonance images. Neuroimage 2012;59:3976–3994.
3. Raffelt D, Tournier J-D, Fripp J, et al. Symmetric diffeomorphic registration of fibre orientation distributions. Neuroimage 2011;56:1171–1180.
4. Raffelt D, Smith R, Ridgway G, et al. Connectivity-based fixel enhancement: Whole-brain statistical analysis of diffusion MRI measures in the presence of crossing fibres. Neuroimage 2015;117:40–55.
5. Smith R, Tournier J-D, Calamante F, et al. SIFT: Spherical-deconvolution informed filtering of tractograms. Neuroimage 2013;67:298–312.
Figures