4509
Optimized Fast High-Resolution Whole-Body Imaging Protocols for Clincial Oncologic PET/MRI1Stanford University, Stanford, CA, United States, 2GE Healthcare
Synopsis
We present a fast sequence with high diagnostic accuracy to move towards high-throughput clinical whole-body cancer staging using PET/MRI. Ferumoxytol-contrast enhanced T1-weighted LAVA-Flex is acquired at 16s/bed, at a voxel size of 3.4x1.5x1.9mm. This contrast-enhanced sequence offers superior vessel contrast and resolution to existing T1 co-registration modalities that are most commonly based on MR attenuation correction sequences or longer T1-weighted sequences. Integration of this sequence in a clinical protocol bears the promise of dramatically accelerated whole-body cancer staging with durations <20min, thus offering similar exam times to the more radiation-invasive alternative of PET/CT.
Introduction
PET/MRI offers simultaneous multimodal imaging acquisition with reduced radiation exposure compared to PET/CT, which is specifically significant to pediatric imaging. However, long MR acquisition times and high cost still hinder acceptance among clinicians. Fast acquisition of whole-body MR protocols of <20min with reliable oncological diagnostic detectability is therefore pivotal for the success of PET/MRI as a high-throughput cancer staging modality1,2. Routinely, Dixon T1 sequences also serving for MR attenuation correction (MRAC) are used as a fast (<20s/bed) anatomical co-registration modality but typically suffer from low resolution. Alternate protocols use T1 fast spin echo (FSE) and half Fourier-acquisition turbo spin-echo (HASTE), requiring 45-60s/bed1. Current T2 sequences mostly require longer scan times of 1-3min/bed. Here, we present a fast breath-hold sequence for co-registration with 18F-FDG PET data as an alternative to conventional PET/CT: a ferumoxytol-enhanced 3D FSPGR T1-weighted Dixon (LAVA-Flex) sequence, acquired at 16s/bed position, which enables co-registered images with excellent T1 contrast and tumor delineationMethods
A PET/MR scanner (Signa, GE Healthcare, Waukesha, WI) was used with a body coil in a whole-body acquisition to test the performance of the proposed protocol. Ferumoxytol-enhanced images were acquired in ten patients using the following sequences: high resolution LAVA-Flex (voxel size = 3.4x1.5x1.9mm and TE/TR/FA=1.7/2.3/15; as well as the more conventionally used low-resolution LAVA-Flex (MRAC) sequence (voxel size = 4.1x2.6mm). The SNR of the two sequences were compared with a statistical t-test. We also compared the spatial resolution and acquisition times of these approaches with other co-registration techniques selected from the literature, including STIR and FSE1,2.Results
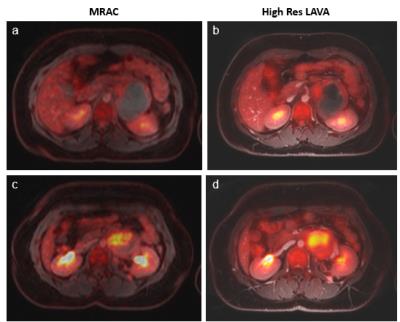
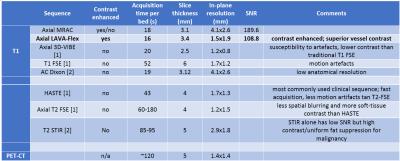
PET/MR images acquired on a patient with Ewing Sarcoma are shown in an anatomical overlay for both the MRAC (Fig. 1a,c) and high-resolution LAVA-Flex sequences (Fig. 1b,d). Table 1 compares relevant image parameters of our proposed sequences to a select set of protocols in the literature.
Scan time reduction: T1 LAVA-Flex can be acquired within 16s, i.e. in a similar duration as the MRAC (18s) and significantly faster than conventional T1 sequences, including FSE (52s)1.
Increased in-plane anatomical resolution: High-resolution LAVA-Flex images are acquired at an in-plane resolution of 1.5x1.9mm, yielding a large improvement in image quality and diagnostic value over MRAC (4.1x2.6mm). Voxel sizes for other, longer sequences are variable and are shown in Table 1 for comparison.
Increased SNR: The SNR between tumor and background signal in a population of 10 patients aged 15 to 23 years with sarcoma were compared between the high-resolution LAVA-Flex and the MRAC sequence and rated with a t-test. The mean SNR was statistically significantly higher for the high-resolution LAVA-Flex sequence (mean SNR = 189.6 ± 20.5) as compared to the MRAC sequence (mean SNR = 108.8 ± 22.7) (p < 0.005).
Discussion
Our proposed protocol can significantly accelerate the total exam time for PET/MR studies. A 2min PET acquisition per bed position easily encompasses both T1 high-resolution LAVA-Flex and MRAC sequences, resulting in an overall scan time of <20min with 5-7 bed positions. This reduction in whole-body scan time provides an important opportunity for tailored PET/MR tumor staging – and is particularly promising for pediatric patients to ensure high image quality1, time and cost efficiency2-4,7,8, and to minimize sedation times5. Previous PET/MR studies in pediatric patients evaluated the head to midthighs in 60 and 45min, respectively, using T2-weighted sequences2,6,8. Using ferumoxytol as a contrast agent, we covered the same area in <20min, with better vessel delineation, and anatomical information more comparable to PET/CT. In addition, the shortened whole-body acquisition time allows for additional dedicated sequences – in particular unenhanced STIR and LAVA. We are currently striving to optimize all other sequences (including T2-weighted FSE and DWI) in an effort to reduce the overall scan time/bed.Conclusions
We presented a fast T1-weighted (LAVA-Flex) sequence for accelerated whole body 18F-FDG-PET cancer staging with whole-body acquisition times of <20 minutes. We anticipate an accelerated push for the PET/MRI modality towards clinical acceptance as a low-radiation alternative to PET/CT with the added flexibility of MRI as a largely versatile diagnostic tool.Acknowledgements
This work was supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, grant number R01 HD081123-01A1.References
[1] Ishii S, et al., Jpn J Radiol (2016). doi:10.1007/s11604-016-0584-9; [2] Martinez-Moeller et al., J Nucl Med 53(9):1415-26 (2012); [3] Ruangwattanapaisarn et al., J Magn Reson Imaging. 42(6):1747-68 (2015). [4] Ciet P, et al., Pediatr Radiol 45:1901-15 (2015). [5] Hirsch FW et al., Pediatr Radiol 43:860-75 (2013). [6] von Schulthess GK et al., J Nucl Med 55:19S-24S (2014). [7] Martinez-Moeller A et al., J Nucl Med 53:1415-26 (2012). [8] Aghighi M et al., Eur Radiol (2016).Figures