4495
Intrinsic susceptibility MRI detects phenotypic alteration induced by a potent anaplastic lymphoma kinase inhibitor in a transgenic model of neuroblastoma1Division of Radiotherapy and Imaging, The Institute of Cancer Research, London, London, United Kingdom, 2Division of Cancer Therapeutics and Division of Clinical Studies, The Institute of Cancer Research, London, London, United Kingdom
Synopsis
In this study we demonstrate that the transverse relaxation rate R2* affords a biomarker of response to a potent anaplastic lymphoma kinase inhibitor in the Th-ALKF1174L/Th-MYCN genetically engineered murine model of neuroblastoma, a childhood cancer of the nervous system
Introduction / Purpose
The clinical outcome of children with high-risk neuroblastoma, a cancer of the developing sympathetic nervous system, remains low. All neuroblastoma survivors have high-risk of developing long-term disabilities and life-threatening conditions caused by current treatments. Better and safer therapies are urgently needed.1 The use of pharmacodynamic (PD) biomarkers has accelerated the clinical development of novel therapeutics against adult cancer. Conventional PD biomarkers necessitate access to post therapy biopsies, which is often not possible in the pediatric population. Therefore non-invasive alternatives, such as imaging biomarkers must be pursued.
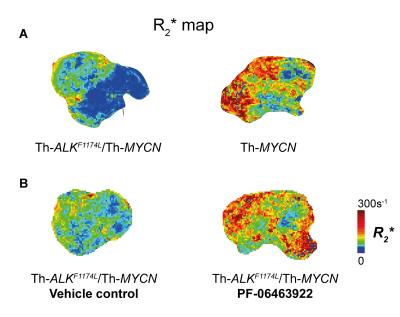
The discovery of aberrations in the anaplastic lymphoma kinase (ALK) gene in 15% of neuroblastoma cases provided a new druggable target against neuroblastoma. Unfortunately the most common mutation, ALKF1174L, which is usually associated with amplification of MYCN, the most established marker of poor prognostic in neuroblastoma, conferred resistance to the promising small molecule ALK inhibitor crizotinib by constitutively activating ALK kinase. We have previously demonstrated that the Th-ALKF1174L/Th-MYCN transgenic model of neuroblastoma mirrors resistance to crizotinib.2 Tumors in the Th-ALKF1174L/Th-MYCN model do not present with the characteristic MYCN-driven neuroblastoma hemorrhagic phenotype, which translates to a marked difference in MRI phenotype characterized by a significantly slower tumor transverse relaxation rate R2* compared with tumors arising in the Th-MYCN model.3 In this prospective study we hypothesised that successful inhibition of ALK with the potent third generation ALK inhibitor, PF-064639224, in the Th-ALKF1174L/Th-MYCN model would induce a shift towards the characteristic hemorrhagic tumor phenotype of the Th-MYCN mouse model. Quantitation of R2* could thus potentially provide a biomarker of response to PF-06463922.
Methods
All experiments were performed in accordance with the UK Home Office Animals (Scientific Procedures) Act 1986 and the United Kingdom National Cancer Research Institute guidelines for the welfare of animals in cancer research.5
Th-ALKF1174L/Th-MYCN Mice with abdominal neuroblastoma were identified by palpation. On day 1, mice were treated daily with 10mg/kg PF-06463922 (Pfizer) p.o. (n=8) or vehicle (n=7). Anatomical MRI was performed on day 0 and weekly until study endpoint. R2* was measured at study endpoint (palpation score).
MRI studies were performed on a 7T Bruker MicroImaging system using a 3cm birdcage volume coil. T2-weighted axial images were used for determining tumor volumes and, following optimization of the local field homogeneity (Fastmap), to plan the R2* measurement (multi-gradient echo sequence, FOV 3x3cm2, 128x128 matrix, TE=6-30ms, 8 TE, TR=200ms, NEX=8), as previously described.3
Results
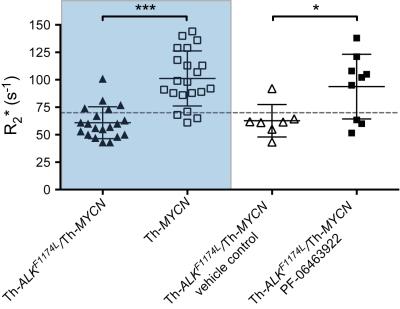
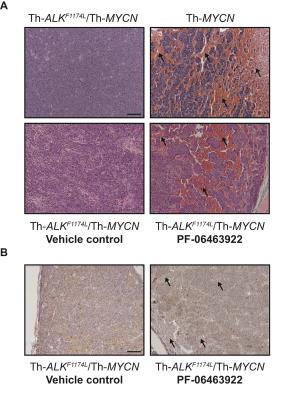
Treatment with PF-06463922 significantly delayed tumor growth in the Th-ALKF1174L/Th-MYCN model, providing two weeks survival advantage. There was no significant difference in MRI-derived tumor volume between the PF-06463922 and vehicle control treated cohorts (1808 ± 149mm3 and 1757 ± 221mm3 respectively). Tumors in PF-06463922-treated mice exhibited a relatively fast R2* at the tumor periphery, with median R2* rates significantly faster than tumors in the vehicle treated cohort, and comparable to the fast tumor R2* rates previously reported in the Th-MYCN mice (Fig.1 & 2)3. H&E staining revealed the presence of blood lakes at the periphery of PF-06463922-treated tumors in regions where ALK is expressed (Fig 3.)Discussion
This study demonstrates that the potent ALK inhibitor PF-06463922 induced a phenotypic change in tumors arising in the Th-ALKF1174L/Th-MYCN transgenic mice that more closely resembled the characteristic hemorrhagic tumor phenotype of the Th-MYCN mouse model. We are currently assessing the phosphorylation status of ALK and downstream target pathways to confirm that the inhibiton of ALK activity, previously demonstrated at day 7 of treatment4, is retained at the endpoint of this study.
R2* potentially affords a biomarker of response to novel therapeutic strategies targeting ALK, such as PF-06463922. Quantification of R2* is rapid and completely noninvasive, and based on our previous work3, has already been incorporated routinely into early phase MRI-embedded clinical trials of novel ALK therapies for children with neuroblastoma at the Royal Marsden Hospital (UK).
Finally, this study further highlights the role of ALK in neuroblastoma vascular morphogenesis and architecture.
Conclusion
Treatment-induced increases in native tumor R2* is a biomarker of response to the potent ALK inhibitor PF-06463922 in the Th-ALKF1174L/Th-MYCN model of neuroblastoma.Acknowledgements
We acknowledge support from The Institute of Cancer Research Cancer Research UK and EPSRC Cancer Imaging Centre, in association with the MRC and Department of Health (England) grant C1060/A10334, NHS funding to the NIHR Biomedical Research Centre, a Cancer Research UK Programme Grant (C18339), a Children with Cancer UK project grant and a Children with Cancer UK Research Fellowship.References
1 Barone, G., Anderson, J., Pearson, A. D. et al. New strategies in neuroblastoma: Therapeutic targeting of MYCN and ALK. Clin Cancer Res. 2013; 19(21): 5814-5821.
2 Berry, T., Luther, W., Bhatnagar, N. et al. The ALK(F1174L) mutation potentiates the oncogenic activity of MYCN in neuroblastoma. Cancer Cell. 2012; 22(1): 117-130.
3 Jamin, Y., Glass, L., Hallsworth, A. et al. Intrinsic susceptibility MRI identifies tumors with ALKF1174L mutation in genetically-engineered murine models of high-risk neuroblastoma. PLoS One. 2014; 9(3): e92886.
4 Guan, J., Tucker, E. R., Wan, H. et al. The ALK inhibitor PF-06463922 is effective as a single agent in neuroblastoma driven by expression of ALK and MYCN. Dis Model Mech. 2016; 9(9): 941-952.
5 Workman, P., Aboagye, E., Balkwill, F. et al. Guidelines for the welfare and use of animals in cancer research. Br J Cancer. 2010; 102(11): 1555-1577.
Figures