4493
Early Detection of Glioblastoma Multiforme by the Magnetic Susceptibility Effect from Deoxyhemoglobin1Chemistry and Biochemistry, UCLA, Los Angeles, CA, United States
Synopsis
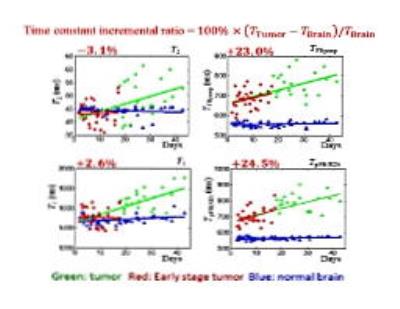
Early detection of high-grade malignancy, such as glioblastoma multiforme (GBM), using new contrast mechanism and enhanced MRI techniques significantly increases not only the treatment options available, but also the patients’ survival rate. For this purpose, the local magnetic-field gradient variations due to irregular water contents and deoxyhemoglobin concentration in early GBM is detected sensitively to provide the needed cancer contrast. Statistical results (N=22) for in vivo orthotopic xenografts GBM mouse models at various cancer stages validate the superior contrast and robustness of this approach (tumor time constant differs from that of the healthy brain tissue by +24%) towards early GBM detection than conventional T1-weighted (+2.6%) and T2-weighted images (-3.1%). This novel approach provides 4-8 times of improvements in early GBM tumor contrast, as measured by "tumor to normal tissue contrast", “contrast-to-noise ratio” (CNR) or “Visibility”.
Purpose
Early detection of high-grade malignancy, such as glioblastoma multiforme (GBM), using new contrast mechanism and enhanced MRI techniques significantly increases not only the treatment options available, but also the patients’ survival rate. For this purpose, the local magnetic-field gradient variations due to irregular water contents and deoxyhemoglobin concentration in early GBM is sensitively detected by active-feedback electronic devices and nonlinear spin dynamics to provide the needed cancer contrast.
Methods
Physiological evidences show that the concentration of deoxyhemoglobin, a paramagnetic molecule, is lower in GBM due to anemia and edema. As GBM cells grow very rapidly, there are usually regional anemia in the brain tumor. GBM cells also increase the vascular endothelial growth factor secretion, weaken the blood brain barrier junctions, and therefore cause excess accumulation of water. Regional anemia and excess accumulation of water cause a local lower concentration of deoxyhemoglobin and weaker local magnetic field gradient. Thus late stage GBM is able to create a contrast in conventional MRI. Since anemia and edema usually emerge even at the early stage of tumor growth, we take advantage of the slightly lower concentration of deoxyhemoglobin at the tumor, compare to the normal brain tissue, and develop new techniques in MRI, termed “Active-Feedback MRI”, to sensitively detect the magnetic susceptibility effect from deoxyhemoglobin for GBM at early stage.
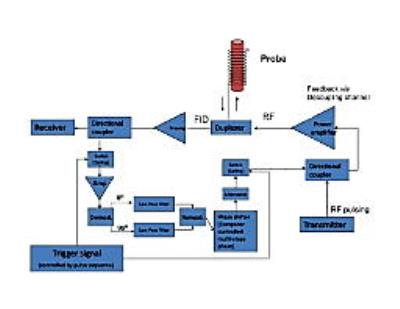
The general principles of the “Active-Feedback MRI” can be found in our publications [1-6] (and references therein). Here, its specific applications to early GBM detection were developed and demonstrated. In essence, the enhanced GBM contrast arises from “selective self-excitation” and “fixed-point dynamics” generated by the bulk water 1H under active feedback fields. [7,8] (i) First, an active-feedback electronic device was home-built to generate feedback fields from the received FID current, as shown in Fig. 1. The device is to filter, phase shift, and amplify the signal from the receiver coils and then retransmit the modified signal into the RF transmission coil, with adjustable and programmable feedback phases and gains. The MR console computer can execute the active-feedback pulse sequences to control the trigger signal, feedback phase/gain, and the duration of the feedback fields, allowing us to utilize the active feedback fields in novel ways. (ii) Next, an active-feedback pulse sequence was developed for early GBM detection, as shown in Fig. 2. Essentially, it is a phase-cycled repeating block of [cw-pi-cw], where active-feedback field is also on during the cw (continuous wave) pulse to enhance the contrast originated from local magnetic-field gradient variations due to irregular water contents and deoxyhemoglobin concentration in early GBM.
Results
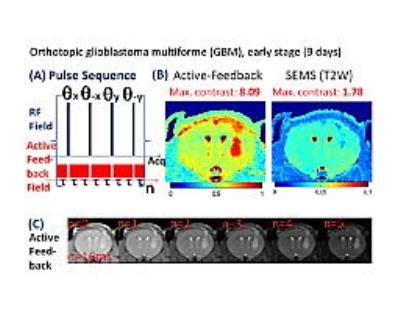
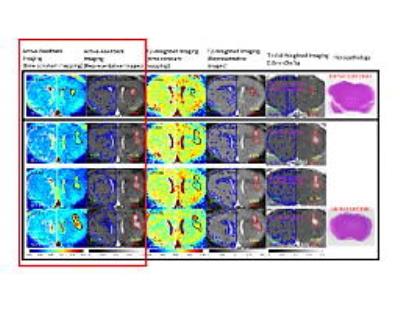
Stage-1 orthotopic GBM mouse models infected with human U87MG cell line were imaged. Representative results from 5 mice were shown in Fig. 3. While T2-parameter images (3rd column), T2-weighted images (4th column), and T1-Gd-weighted images (5th column) could not successfully locate the early GBM tumor, our active-feedback fixed-point images (2nd column) and decay constant mapping (1st column) successfully highlight the early GBM tumor with a close correlation with histopathology (6th column). Statistical results (N=22) for in vivo orthotopic xenografts GBM mouse models at various cancer stages validate the superior contrast and robustness of this approach (tumor time constant differs from that of the healthy brain tissue by +24%) towards early GBM detection than conventional T1-weighted (+2.6%) and T2-weighted images (-3.1%), as shown in Fig. 4.Conclusion
Statistical results (N=22) show that this new approach provides 4-8 times of improvements in early GBM tumor contrast, as measured by "tumor to normal tissue contrast", “contrast-to-noise ratio” (CNR) or “Visibility”.Acknowledgements
This work was supported by the Camille and Henry Dreyfus Foundation (TC-05-053), National Science Foundation (DMS-0833863, CHE-1112574, and CHE-1416598), Hirshberg Foundation for Pancreatic Cancer Research, and Taiwan Ministry of Science and Technology (NSC 100-2113-M-002-008, NSC 101-2113-M-002-018, and MOST 103-2923-M-002-006).References
[1] Science 290, 118 (2001) [2] Magn. Reson. Med. 56, 776 (2006) [3] Magn. Reson. Med. 61, 925 (2009) [4] J. Phys. Chem. B 110, 22071 (2006) [5] Curr. Pharm. Des. 21, 1 (2015) [6] Biomaterials 37, 436 (2015) [7] J. Magn. Reson. 248, 19 (2014) [8] Magn. Reson. Med. 74, 33 (2015)
Figures