4492
Application of Cardio-Respiratory Gated High Resolution 3D Balanced SSFP to Liver Tumour Imaging in the Mouse1CRUK/MRC Oxford Institute for Radiation Oncology, University of Oxford, Oxford, United Kingdom
Synopsis
Prospective gating and automatic reacquisition of data corrupted by respiration motion were implemented in 3D balanced steady-state free precession (bSSFP) to provide contrast that enables unambiguous detection of liver tumours in the whole mouse liver with 200 µm isotropic resolution and in scan times that are routinely less than 7 minutes. The method was used for orthotopic tumour burden quantification in 8 female C57BL/6 mice at days 7, 9, and 11 post intra-hepatic injection of MC38-GFP cells, and enabled measurement of tumour volumes less than 1 mm3.
Purpose
The balanced steady-state free precession (bSSFP) scan mode is of particular interest because it is fast and the inherent ‘T2’ contrast causes many pathologies to appear bright. Cardio-respiratory (CR) synchronisation, k-space segmentation and short constant TR have recently been applied to 3D bSSFP to reduce imaging times below what is otherwise achievable with standard techniques such as retrospective gating.1 This abstract demonstrates that the method enables robust imaging of liver tumours in the mouse with 200 µm isotropic resolution in less than 7 minutes and is therefore a suitable tool for the routine screening of disease development and monitoring of in vivo tumour response to therapy.Methods
Anaesthesia was induced with 4% isoflurane in air and maintained with 1-3% isoflurane in a 1:5 O2:air mixture for surgery and MRI. 1x104 to 5x105 MC38-GFP tumour cells were injected directly into the liver of 8 female C57Bl/6 mice to generate orthotopic tumours.2 MRI was performed at days 7, 9, and 11 post inoculation at 7.0 T (Varian VNMRS), using a 30 mm long 25 mm ID quadrature birdcage (Rapid Biomedical). Respiration was monitored using a pressure balloon. ECG needles were placed subcutaneously in the chest. CR-synchronised bSSFP scans were performed volumetrically with automatic and immediate reacquisition of data corrupted by respiration motion. Scan parameters were TR 2.8 ms, TE 1.4 ms, RF hard pulse 16 µs, FA 30º, FOV 51.2×25.6×25.6 mm3, matrix 256×128×128, and 32 k-lines per R-wave to give a single 3D data set with 200 µm isotropic resolution in less than 105 s. bSSFP banding artefacts were robustly removed with combination of four phase-cycled images acquired in less than 7 minutes in total using an elliptical signal model.3 CR-synchronised RF and gradient spoiled 3D gradient echo scans were acquired with identical scan parameters apart from TE 1.15 ms, FA 8º, and the acquisition of 4 identical repeats to give a comparative scan of essentially the same duration. The bSSFP scan is marginally longer to enable each phase-cycled image to reach steady-state before the start of acquisition. Liver tumour volumes were calculated using the threshold-based automatic segmentation provided by ITK-SNAP.4Results
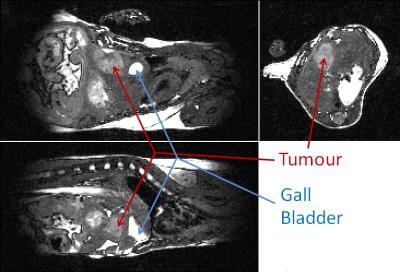
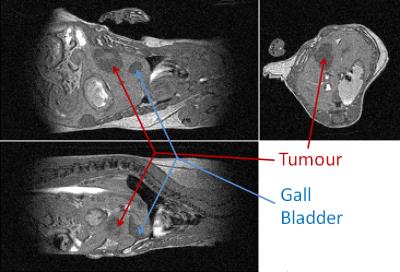
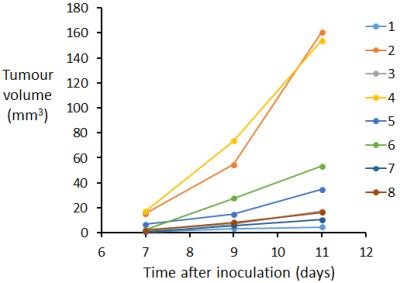
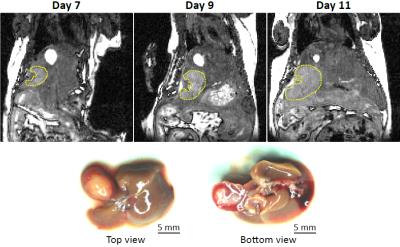
Examples of orthogonal views through the tumour are shown for mouse 4 at 9 days post intra-hepatic injection in Figures 1 and 2 for the bSSFP and gradient echo scan modes respectively. Figure 3 shows the tumour volumes of 8 mice which varied from 0.824 mm3 to 160.7mm3. Tumour growth was observed between successive observations in all 8 mice. Figure 4 shows slices through the tumour in bSSFP scans of mouse 4 at 7, 9 and 11 days post intra-hepatic injection. Photographs of the excised liver confirm the presence of the tumour and the bottom view replicates the tumour shape observed in the MRI slices.Discussion
In both scan modes the automatic and immediate reacquisition of data corrupted by respiration motion robustly eliminates respiration motion artefact. Contrast is generated over a timescale of up to several T1 but maintained with a timescale of TR such that synchrony with the cardiac R-wave can always be achieved to within one TR which is 2.8 ms in this instance. It is evident that application of the bSSFP scan mode enables unambiguous delineation of tumour within the liver. Although the gradient echo scan is more robust with respect to movement of spins since phase coherence of magnetisation is only required during one TE, pure T1 contrast is not able to distinguish between tumour and, for example, gall bladder. Due to the lack of accurate and fast in vivo orthotopic tumour volume quantification methods, most therapy response studies are done in subcutaneous models, which do not entirely mimic the normal tumour microenvironment. It has been demonstrated that the bSSFP scan mode allows rapid assessment of orthotopic liver tumour volumes. Furthermore the method would enable quantification of response to anti-cancer agents and/or other therapies in vivo, and could be applied to a variety of other disorders.Conclusion
Cardio-respiratory gated 3D bSSFP enables unambiguous detection of orthotopic liver tumours in the mouse with 200 µm isotropic resolution and in scan times that are routinely less than 7 minutes.Acknowledgements
The work presented was supported financially by Cancer Research UK (CRUK grants C5255/A12678, C2522/A10339), the Engineering and Physical Sciences Research Council (EPSRC grant C2522/A10339) and the Medical Research Council Unit Grant for the Oxford Institute for Radiation Oncology.References
1. Kinchesh P, Gilchrist S, Gomes AL, Kersemans V, Beech J, Allen D, Smart S. Accelerated imaging of the mouse body using k-space segmentation, cardio-respiratory synchronisation and short, constant TR: Application to b-SSFP. Proc Intl Soc Mag Reson Med 2016;24:1825.
2. Rao Q, You A, Guo Z, Zuo B, Gao X, Zhang T, Du Z, Wu C, Yin H. Intrahepatic tissue implantation represents a favorable approach for establishing orthotopic transplantation hepatocellular carcinoma mouse models. Plos One 2016;11:e0148263.
3. Xiang Q-S, Hoff MN. Banding artifact removal for bSSFP imaging with an elliptical signal model. Magn Reson Med 2014;71:927-933.
4: Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006;31:1116-1128.
Figures