4491
Radiological comparisons of 3D and 2D-cell culture derived tumours in an orthotopic mouse xenograft model of paediatric glioblastoma1The Institute of Cancer Research, London, United Kingdom, 2Department of Oncology, Hospital Sant Joan de Déu, Barcelona, Spain
Synopsis
Primary patient-derived paediatric glioblastoma (pGBM) cells, cultured under conditions designed to maintain stem-like phenotypes, provide valuable platforms for pre-clinical research. Culturing tumour cells in suspension (3D) or adherent on laminin (2D) may influence their growth behaviour in vivo. Longitudinal, anatomical MRI showed that orthotopic HSJD-GBM-001 xenografts derived from 3D-cultured cells grew significantly faster than those from 2D-culture. Infiltrative tumour growth, maintenance of the blood brain barrier and high ADC, T1 and T2, associated with histopathologically confirmed tumour-oedema, were observed in both cohorts. The study highlights the influence of in vitro cell culture conditions on in vivo tumour growth characteristics.
Introduction
Paediatric glioblastoma (pGBM) is a highly invasive childhood brain tumour that has seen no improvement in patient survival for decades1. Advances in new treatments have been hampered by a number of factors, including the lack of appropriate cell lines and pre-clinical models that faithfully replicate human disease.
Primary patient-derived pGBM cell lines are propagated under stem cell culture conditions, either in suspension as neurospheres (3D), or adherent on laminin (2D), a fibrous protein of the extracellular matrix. 3D and 2D cultured cells differ in cell-cell versus cell-matrix interactions, access to nutrients from the culture medium, and other factors, which may play a role in determining the self-renewal capacity and doubling times of the cells. An understanding of how these in vitro differences impact on in vivo pGBM tumour growth behaviour has not yet been established, but could guide more effective in vivo tumour modelling in pre-clinical trials.
We aimed to perform radiological comparisons between orthotopic tumours derived from patient-derived HSJD-GBM-001 pGBM cells grown in 3D and 2D cell culture.
Methods
Patient-derived HSJD-GBM-001 cells were expanded in either 3D or 2D using stem cell culture medium, in the absence of serum and presence of growth factors (human (h)-EGF, h-FGF, h-PDGF-AA and h-PDGF-BB). 2.5x105 cells were orthotopically injected in the right frontal lobe of 6-week old nude mice.
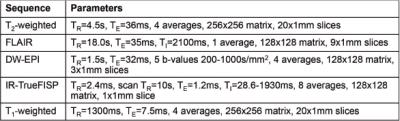
MRI was carried out on a 7T Bruker horizontal bore microimaging system using a 3cm birdcage coil over an axial 2.5cmx2.5cm field-of-view. Longitudinal, anatomical T2-weighted MRI and FLAIR imaging were applied from day 19 post-injection every 14 days to localise the tumour, and establish tumour growth characteristics. At study end, diffusion-weighted (DW), inversion recovery (IR) trueFISP, and T1-weighted (prior to and following intravenous Gd-DTPA (0.1mmol/kg Magnevist, Schering) administration) MR images were also acquired. Sequence parameters are shown in Table 1.
Parametric apparent diffusion coefficient (ADC), T1, and T2 maps were generated by fitting the data on a pixel-by-pixel basis with a Bayesian maximum a posteriori algorithm2 using in-house software. Tumour regions-of-interest (ROIs) were analysed to quantify median ADC, T1, and T2 values.
Serial 4µm sections were cut from formalin-fixed paraffin-embedded brain tissue, and stained with haematoxylin and eosin (H&E), or immunohistochemically processed for the detection of human nuclear antigen (HNA) to identify tumour cells, or Ki67 to assess tumour cell proliferation.
Results
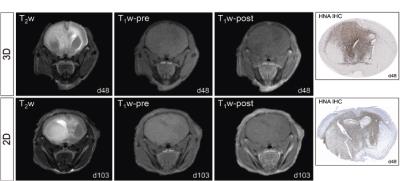
HSJD-GBM-001 mouse xenograft tumours derived from both 3D and 2D cultured cells presented as hyperintense lesions with indistinct boundaries on T2-weighted MRI (Figure 1). Following intravenous Gd-DTPA administration, no signal enhancement was apparent in the brains of either cohort. HNA immunohistochemistry revealed abundant human tumour cell nuclei diffusely distributed throughout the brains of all mice.
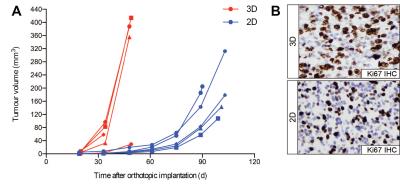
HSJD-GBM-001 tumours derived from cells cultured in 3D grew significantly faster than those from 2D culture (mean tumour doubling times 4.3 days and 10.4 days, respectively, unpaired Student’s t-test p=0.008) (Figure 2A). Quantitative analysis of Ki67 staining within tumour-bearing regions revealed increased cell proliferation in tumours derived from 3D culture compared to 2D culture, corroborating the growth advantage observed on anatomical MRI (Ki67 scores 76% and 59%, respectively, p=0.004) (Figure 2B).
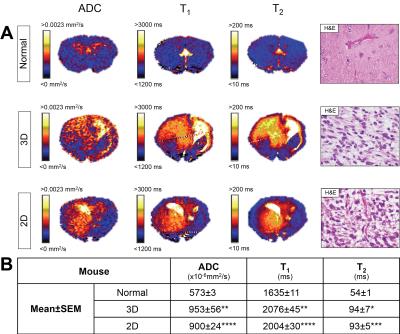
Whole brain parametric ADC, T1, and T2 maps, and quantification from tumour ROIs, from HSJD-GBM-001 bearing mice showed heterogeneous elevation of all parameters compared to non-tumour-bearing (normal) brain tissue (Figure 3). H&E staining revealed abnormal cell nuclei and abundant oedema in both tumour cohorts. There was no significant difference in ADC, T1 and T2 between tumours derived from 3D or 2D cultured cells.
Discussion
HSJD-GBM-001 tumours derived from 3D culture grew significantly faster than tumours from 2D culture, tumour cell proliferation was also higher in these tumours, indicating that 3D-cultured HSJD-GBM-001 cells may prime the in vivo stem cell self-renewal capacity more effectively, causing an in vivo tumour growth advantage.
Infiltrative tumour growth patterns were observed in both cohorts, and the blood brain barrier (BBB) was intact as shown by the absence of post-contrast enhancement. HSJD-GBM-001 tumours were highly infiltrative, and did not form a typical tumour mass, exerting pressure on the delicate endothelial cell alignment resulting in a disruption of the BBB.
Elevated ADC, T1, and T2 were consistent with the tumour-associated oedema identified on H&E stained sections, a feature commonly observed in the clinic3.
Conclusion
Longitudinal, anatomical MRI demonstrated that cell culture conditions influence the in vivo tumour growth rate of patient-derived HSJD-GBM-001 cells, indicating that orthotopic engraftment of these cells cultured in 3D provides a promising in vivo model, which could benefit future pre-clinical trials for pGBM.Acknowledgements
We acknowledge the Cancer Research UK (CR-UK) support to the Cancer Imaging Centre at The Institute of Cancer Research (ICR) and Royal Marsden Hospital (RMH), in association with MRC and the Department of Health C1060/A16464, and NHS funding to the NIHR Biomedical Research Centre.References
1. Broniscer A, Gajjar A. Supratentorial high-grade astrocytoma and diffuse brainstem glioma: two challenges for the pediatric oncologist. Oncologist. 2004;9(2):197-206.
2. Boult JKR, Borri M, Jury A et al. Investigating intracranial tumour growth patterns with multiparametric MRI incorporating Gd-DTPA and USPIO-enhanced imaging. NMR Biomed. 2016;29(11):1608-1617.
3. Fangusaro J. Pediatric high grade glioma: a review and update on tumor clinical characteristics and biology. Front Oncol. 2012;2:105.
Figures