4489
Diffusion kurtosis imaging can stratify differentiation of colorectal cancers: a preliminary study1Department of Radiology, Xinhua hospital, Shanghai Jiaotong University School of Medicine, Shanghai, People's Republic of China, 2Department of Radiology, Xinhua hospital, Shanghai Jiaotong University School of Medicine, 3Department of Radiology, Xinhua hospital, Shanghai Jiaotong University School of Medicine, People's Republic of China, 4Philips Healthcare, People's Republic of China
Synopsis
Colorectal cancer (CRC) is the third most common cancer in the world and its incidence is on the rise. Management is particularly challenging technically for surgeon and local recurrence is a common result of treatment failure. Tumor differentiation grade is one of the factors that influence the choice of individual management, then affecting the prognosis. Diffusion kurtosis imaging (DKI) could quantify non-Gaussian behavior of water diffusion and provide more precise information of tissue characteristics. Our study demonstrated that DKI is conducive to distinguishing the various differentiations of CRC models, and reflecting the proliferation indirectly in tumor cells.
Purpose: Colorectal cancer (CRC) is the third most common cancer in the world and its incidence is on the rise 1. Management is particularly challenging technically for surgeon and local recurrence within the pelvis is a common result of treatment failure 2. The prognostic factors for patients with rectal cancer included the degree of tumor invasion into and beyond the bowel wall, the number of lymph nodes involved, and involvement of the mesorectal fascia, the plasma carcinoembryonic antigen level, tumor differentiation grade, and the lymphangiovascular invasion 3. These factors are important for the choice of individual management. Diffusion kurtosis imaging (DKI) is an advanced diffusion weighted imaging (DWI) model, which could quantify non-Gaussian behavior of water diffusion and provide more precise information of tissue characteristics, such as complexity of tissue microstructural environments4. Therefore, the aim of our study was to explore the value of DKI in stratifying differentiations of CRC xenograft models.
Methods: Poorly-differentiated CRC cells of HCT116, intermediate-differentiated CRC cells of SW480, and well-differentiated CRC cells of HT29 were subcutaneously injected into the right hind flank to establish the xenograft models with various differentiations (n=10 per group). When the tumor volume was about 500 mm3, conventional T2-weighted magnetic resonance imaging (MRI) and DKI sequence were performed on a 3.0T MR system (Ingenia, Philips, Medical System, Best, Netherlands) with an eight-channel receiver coil of 5.0 cm inner diameter. The single-shot spin-echo echo-planar DKI sequence was performed by using tri-directional motion-probing gradients with the b-values of 0, 500, 1000, 1500 and 2000 s/mm2 (TR/TE 3000/59 ms, 4-6 slices, slice thickness 2 mm, no interslice gap, field of view 50×50 mm2, matrix 64×64, parallel imaging factor 3, receiver bandwidth 1047.9 Hz, 19 signal averages and acquisition time of approximately 6.5-13.5 min). The apparent diffusion coefficient (ADC), diffusivity (D), and kurtosis (K) maps were calculated by using in-house software developed in IDL 6.3 (ITT Visual Information Solutions, Boulder, CO) (Fig.1). The ADC calculated by using the standard monoexponential fit, DKI-derived parameters of D and K calculated by performing the voxel-by-voxel analysis on the basis of DKI nonlinear equation were compared by performing by a one-way analysis of variance (ANOVA). The associations between ADC, D, and K and Ki-67 expression were analyzed.
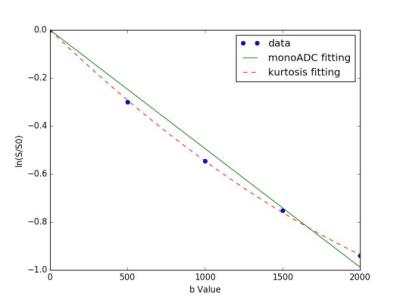
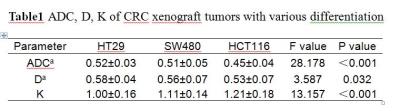
Results: The image quality of conventional MRI and DKI was good. DKI performed a better fitting to the diffusion-weighted signal than the monoexponential ADC (Fig.2). As shown in table 1, with the decrease of the degree of tumor differentiation, ADC and D were significantly decreased in HT29, SW480, and HCT116 xenograft models (P<0.001, P=0.032, respectively) while K was significantly increased (P<0.001). For differences between groups, ADC of HCT116 group was significantly lower than those of SW480 group (P<0.001) and HT29 (P<0.001). D of HCT116 group was significantly lower than that of HT29 group (P=0.009). While there were no significant differences for ADC and D between SW480 and HT29 group (P=0.200, P=0.291, respectively). For K, there were significant differences between HCT116 and SW480 group (P=0.011), between HCT116 and HT29 group (P<0.001), and between SW480 and HT29 group (P=0.013). The coefficients of variation for diffusivity and kurtosis were 7.3% and 4.9%, respectively. The diffusion and kurtosis were significantly correlated with Ki-67 expression(r=0.85,P<0.05;r=0.72,P<0.03).
Discussion: DKI model could quantify the non-Gaussian behavior of water diffusion, which is more accurate and sensitivity in assessing the complexity of tissue microstructural environments, compared with the standard monoexponential model. In the xenograft models with various differentiations, cell dedifferentiation could lead to higher restriction of water diffusion and more heterogeneity. The changes of tissue microstructural environments could be reflected by the DKI-derived parameters of D and K. The Ki-67 antigen is used to evaluate the proliferative activity of tumors. The different proliferative activity of tumors could lead to the different extent of water diffusion and heterogeneity, which could also be reflected by D and K.
Conclusion: In our preliminary study, DKI is conducive to distinguishing the various differentiations of colorectal cancer models, and reflecting the proliferation indirectly in tumor cells.
Acknowledgements
No acknowledgement found.References
1. Cho EY, Kim SH, Yoon JH, et al. Apparent diffusion coefficient for discriminating metastatic from non-metastatic lymph nodes in primary rectal cancer. Eur J Radiol. 2013; 82(11): e662–8.
2. Ucar A, Obuz F, Sokmen S, et al. Efficacy of high resolution magnetic resonance imaging in preoperative local staging of rectal cancer. Mol Imaging Radionucl Ther. 2013; 22(2):42–8.
3. Akashi M, Nakahusa Y, Yakabe T, et al. Assessment of aggressiveness of rectal cancer using 3-T MRI: correlation between the apparent diffusion coefficient as a potential imaging biomarker and histologic prognostic factors. Acta Radiol. 2014; 55(5):524-31.
4. Rosenkrantz AB, Sigmund EE, Winnick A, et al. Assessment of hepatocellular carcinoma using apparent diffusion coefficient and diffusion kurtosis indices: preliminary experience in fresh liver explants. Magn Reson Imaging. 2012; 30(10):1534-1540.
Figures

HT29: well-differentiated colorectal cancer,
SW480: intermediate-differentiated colorectal cancer,
HCT116: Poorly-differentiated colorectal cancer
a *10-3mm2/s