4485
Imaging Collagenase-Induced Changes in the Mechanical Phenotype of Orthotopic BT474 Breast Cancer Xenografts Using Magnetic Resonance Elastography1Division of Radiotherapy & Imaging, The Institute of Cancer Research, London, United Kingdom, 2Division of Imaging Sciences and Biomedical Engineering, King's College London, King's Health Partners, St. Thomas' Hospital, London, United Kingdom
Synopsis
We tested the hypothesis that MRE can inform on collagenase-induced matrix degradation in orthotopic BT474 breast carcinoma xenografts in vivo. An acute reduction in the absolute value of the complex shear modulus |G*| was detected in tumour just 5 hours after collagenase administration, mostly likely a consequence of both collagen degradation and reduction of interstitial fluid pressure. The study highlights the utility of MRE-derived quantitation of tumour viscoelasticity for monitoring the response of stromal rich tumours to modification of the extracellular matrix.
Introduction / Purpose
In most solid tumours, increased deposition of collagen and other extracellular matrix (ECM) components contributes to elevated interstitial fluid pressure (IFP) and represents a physical barrier for the efficient delivery of drugs.1 Stroma-targeted therapies have been shown to improve the delivery of conventional chemotherapy, resulting in enhanced treatment efficacy.2-5 Intravenous administration of collagenase led to significant acute reduction in tumour IFP in several tumour models.2,3,6 Magnetic resonance elastography (MRE) is being exploited for the non-invasive imaging and quantitation of tissue viscoelastic properties, and its application for the diagnosis of breast cancer is being investigated.7,8 Using MRE, we have demonstrated that stromal-dense and collagen-rich orthotopically propagated BT474 breast cancer xenografts presented with a very stiff mechanical phenotype in vivo.9 In this study we tested the hypothesis that the degradation of the collagen matrix by exogenously administered collagenase would induce a significant reduction in the viscoelastic properties of BT474 tumours measured with MRE.Methods
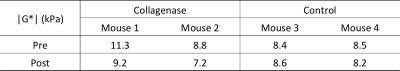
All experiments were performed in accordance with the UK Animals (Scientific Procedures) Act 1986. Orthotopic BT474 breast carcinomas were propagated in the mammary fat pad of female nude mice previously implanted with slow release estradiol pellets (5x106 cells in 100 µl 50% matrigel). Collagenase (Sigma-Aldrich, type I, from clostridium histolyticum) was administered intravenously via a lateral tail vein (0.5 mg/kg; 0.1% w/v in PBS (1 mg = 125 collagen digestion units)).6 MRE data were acquired, as previously described,9 24 hours before and 5 hours after treatment with collagenase (n=2) or without treatment (n=2). Average tumour size was 149 mm3. Parametric maps of absolute value of the complex shear modulus |G*| (G* = Gd + iGl, Gd: elasticity, Gl: viscosity, all in kPa) were calculated using in-house software, reporting volumetric mean values from ROIs covering the entire tumour over 6 slices, sampling at a slice interval of 300 µm.Results
BT474 tumours revealed a heterogeneous distribution of markedly elevated |G*| values at baseline (Fig.1 & Table 1), relative to 5.24 kPa for SW620 colorectal xenografts.10 After 5 hours, treatment with collagenase induced a clear reduction in shear stiffness |G*| (Fig.1 & Table 1) in both treated tumours. In contrast, there was no apparent change in |G*| in the control animals. The mechanical response to collagenase was heterogeneous, with relatively stiffer tumour regions at baseline exhibiting the largest reductions in |G*| post-treatment.Discussion
This MRE study showed that treatment of orthotopic BT474 breast xenografts with collagenase resulted in a reduction in tumour |G*| after just 5 hours. This acute mechanical response to collagenase is most likely a consequence of both degradation of the collagen matrix and a consequent reduction in IFP. Degradation of collagen either lining blood vessels or in the ECM has been associated with a decrease in the complex shear modulus |G*|,11,12 and shown to occur as early as one hour following treatment with collagenase in a human osteosarcoma xenograft model.2 Shear modulus is also related to intravascular and interstitial fluid pressure.13 An increase in |G*|, as measured by MRE, in the cerebrum of normal pressure hydrocephalus patients was associated with an elevation of intracranial pressure.14 Intravenous administration of collagenase induced significant acute reductions in IFP in a range of pre-clinical tumour models.2,3,6Conclusion
Treatment with collagenase leads to an acute and detectable change in the mechanical phenotype measured by MRE in orthotopic BT474 breast xenografts. This preliminary study warrants further investigations to evaluate the potential of MRE-derived viscoelastic biomarkers for assessing breast cancer response to collagenase, or other stroma-targeting strategies, in this model.Acknowledgements
We acknowledge the CRUK and EPSRC support to the Cancer Imaging Centre at ICR in association with MRC and Department of Health C1060/A16464 and NHS funding to the NIHR Biomedicine Research Centre, a Paul O’Gorman Postdoctoral Fellowship funded by Children with Cancer UK, and funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 668039.References
1. Heldin CH, Rubin K, Pietras K, et al. High interstitial fluid pressure - an obstacle in cancer therapy. Nat Rev Cancer 2004; 4: 806-813.
2. Eikenes L, Bruland OS, Brekken C, et al. Collagenase increases the transcapillary pressure gradient and improves the uptake and distribution of monoclonal antibodies in human osteosarcoma xenografts. Cancer Res 2004; 64: 4768-4773.
3. McKee TD, Grandi P, Mok W, et al. Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer Res 2006; 66: 2509-2513.
4. Kultti A, Li X, Jiang P, et al. Therapeutic targeting of hyaluronan in the tumor stroma. Cancers (Basel) 2012; 4: 873-903.
5. Provenzano PP, Cuevas C, Chang AE, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 2012; 21: 418-429.
6. Hassid Y, Eyal E, Margalit R, et al. Non-invasive imaging of barriers to drug delivery in tumors. Microvasc Res 2008; 76: 94-103.
7. Sinkus R, Siegmann K, Xydeas T, et al. MR elastography of breast lesions: understanding the solid/liquid duality can improve the specificity of contrast-enhanced MR mammography. Magn Reson Med 2007; 58: 1135-1144.
8. Sinkus R, Lorenzen J, Schrader D, et al. High-resolution tensor MR elastography for breast tumour detection. Phys Med Biol 2000; 45: 1649-1664.
9. Li J, Jamin Y, Boult JKR, et al. Imaging biomarkers of cell death: a comparison between viscoelasticity and ADC in an orthotopic breast cancer xenograft model. Proc Intl Soc Mag Reson Med 24, 2014; 915.
10. Li J, Jamin Y, Boult JK, et al. Tumour biomechanical response to the vascular disrupting agent ZD6126 in vivo assessed by magnetic resonance elastography. Br J Cancer 2014; 110: 1727-1732.
11. Cox TR, Bird D, Baker AM, et al. LOX-Mediated Collagen Crosslinking Is Responsible for Fibrosis-Enhanced Metastasis. Cancer Res 2013.
12. Newman S, Cloitre M, Allain C, et al. Viscosity and elasticity during collagen assembly in vitro: relevance to matrix-driven translocation. Biopolymers 1997; 41: 337-347.
13. Ricardo L, Paul EB, Assad AO, et al. Coupling between elastic strain and interstitial fluid flow: ramifications for poroelastic imaging. Physics in Medicine and Biology 2006; 51: 6291.
14. Fattahi N, Arani A, Perry A, et al. MR Elastography Demonstrates Increased Brain Stiffness in Normal Pressure Hydrocephalus. AJNR Am J Neuroradiol 2016; 37: 462-467.
Figures