4481
Magnetic Resonance Angiography Shows Increased Arterial Blood Supply Associated with Murine Mammary CancerDevkumar Mustafi1, Abby Leinroth1, Xiaobing Fan1, Erica Markiewicz1, Marta Zamora1, Jeffrey Mueller2, Suzanne D Conzen3, and Gregory S Karczmar1
1Radiology, The University of Chicago, Chicago, IL, United States, 2Pathology, The University of Chicago, Chicago, IL, United States, 3Medicine, the Section of Hematology and Oncology, The University of Chicago, IL, United States
Synopsis
Breast cancer is a major cause of morbidity and mortality in Western women. Tumor neo-angiogenesis may be an MRI-detectable prognostic marker for cancer progression. Clinical practice uses DCE-MRI to detect cancers based on increased blood flow and capillary permeability. However, DCE-MRI requires repeated injections of contrast media; therefore we used time-of-flight MR angiography to measure the number and size of arteries feeding mammary glands with and without cancer, and demonstrated that blood vessels in and near mammary glands grew significantly as invasive cancers developed.
PURPOSE
Breast cancer in humans is associated with increased blood supply and capillary permeability. Typically, mammary vasculature is detected and evaluated using DCE-MRI. While DCE-MRI is the preferred method for clinical detection of human breast cancer, DCE-MRI for serial measurements in mouse models of breast cancer is challenging. It requires repeated injections of contrast media. Therefore we tested non-invasive angiography for serial studies of mouse mammary glands. The goal of the present study is to use time-of-flight (TOF) MR angiography to measure the number and size of arteries feeding mammary glands with and without cancer.METHODS
Nine SV40TAg mice, 18-20 weeks of age, were used. Fast spin echo MR images (multi-slice RARE, TR/TEeffective=4000/20 ms, slice=61, slice thickness=0.5 mm, and in-plane resolution=0.1 mm) of inguinal mammary glands were acquired at 9.4T. For TOF, a flow compensated, gradient echo sequence with a short TR (TR/TEeffective=10/3 ms) was used to maximize inflow effects and depict flowing blood as a bright signal; other parameters were as in RARE. After in vivo MRI studies, the mammary glands were excised for histological processing and hematoxylin and eosin (H&E) staining of slices. H&E slides were then evaluated by an experienced breast pathologist and were classified as normal gland, in situ, or invasive cancers. Based on criteria defined and published previously,1 we identified invasive tumors in the inguinal mammary glands from nine SV40 mice on RARE images. Regions-of-interest (ROIs) were manually traced around mammary cancers. Total tumor volume in each mammary gland was determined by combining the volumes of all cancer ROIs. To determine the volumes of arteries feeding mammary glands, the left and right inguinal mammary gland were manually traced on RARE images first and the resulting ROIs were superimposed on the TOF images. Then thresholds were set to select only those pixels representing blood vessels. Using these pixels, the total volumes of arteries in the left and right mammary glands were calculated. We calculated the volumes of the mammary cancers or blood vessels on each slice by multiplying the total cross sectional area (slice thickness=0.5 mm) in both RARE and TOF images. Then all of the slice volumes were added together to estimate the final tumor and vessel volumes on each side of mammary gland. 3D volume-rendered MR images were then correlated with mammary gland histology to assess both the vessel density and tumor burden. The Pearson correlation test was performed to examine whether there is a linear relationship between mammary cancer volumes and blood volumes.RESULTS and DISCUSSION
Using time-of-flight angiography the smallest detectable artery on volume-rendered images was 0.005 mm3. The average SNR of blood vessels to background in TOF, measured over ROIs, was 61.1±12.0. The blood vessels had significantly higher SNR than muscle (p<0.00035). Blood volume increases with cancer burden, as blood vessels are more abundant in the right gland, particularly beneath the tumor (Figure 1). In the left gland no cancer is evident and no blood vessels are detected. This qualitative relationship was visualized more easily with 3D-rendering (Figure 2). Arteries leading to mammary glands with low or no tumor burden are well organized in a tree-like structure with alternating branches. Arteries leading to glands with higher tumor burdens do not have a regular branching pattern. Instead the blood vessels grow in a clump directed towards the tumor and surrounding tissue. A strong correlation (r=0.79, p˂ 0.0001) was found between increasing tumor volume and blood volume (Figure 3). The results suggest that blood volume in the mammary gland detected by MRI increases by 8.5% when tumor volume doubles (the slope of Figure 3). However, not all of the cancers identified in this study conformed to the approximately linear relationship between blood volume and tumor burden. MRI is consistent with histology; Figure 4 depicts the histology of the mouse shown in Figures 1 and 2. This histology shows that the right gland, Figure 4b, which has a larger tumor, not only has more vessels but also more vascular complexity.CONCLUSIONS
To the best of our knowledge the present study is the first to demonstrate a strong correlation between tumor volume and blood volume in mammary cancer in vivo, completely non-invasively without use of contrast agents. The results demonstrate that TOF-MR angiography can non-invasively monitor changes in vasculature during cancer development in mouse models, without the need to place I.V. lines and inject contrast agents. In addition, the present results provide a rationale for testing this approach in patients, as a way to avoid costs and avoid adverse effects of contrast agents.Acknowledgements
This research is supported by grants from the National Institutes of Health (R01-CA133490 and R01-CA167785), Florsheim foundation, Segal foundation, and VPH prism grant from the European Union. The Lynn S. Florsheim Magnetic Resonance Laboratory subcore of the Integrated Small Animal Imaging Research Resource is partially supported by funds from the University of Chicago Comprehensive Cancer Center from the National Cancer Institute Cancer Center Support Grant P30CA014599.References
1. Mustafi D, Zamora M, Fan X, et al. MRI accurately identifies early murine mammary cancers and reliably differentiates between in situ and invasive cancer: Correlation of MRI with histology. NMR Biomedicine 2015;28(9):1078-1086.Figures
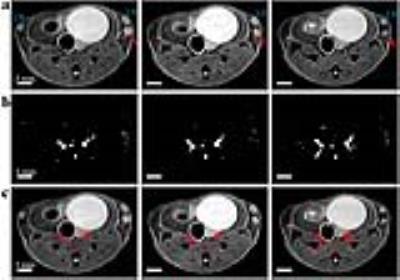
Figure 1. In vivo MR images of a SV40TAg mouse of 19
weeks of age. The top panel shows RARE images
of the three central slices of 0.5 mm in thickness of inguinal mouse mammary
glands. In all images lymph nodes (LN) are labeled and tumors are indicated by
red arrows. The middle panel shows the TOF images of the corresponding slices
as seen in the top panel. The bottom panel shows blood vessels, shown in red as
in TOF images in the middle panel, overlaid on T2W images. Scale bars in all
images are shown.
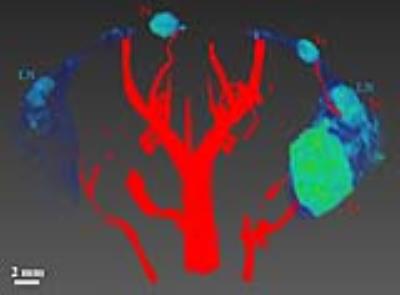
Figure 2. Three dimensional volume-rendered image shows mouse mammary glands and vessel densities of a
SV40TAg female mouse at 18 weeks of age. Only
the ROIs of both inguinal mammary glands are shown in blue-to-green color –
images were acquired with a T2-weighted RARE sequence. Blood vessels
are shown in red – images of blood vessels were constructed from TOF datasets. Blood
vessels in and near the right inguinal gland, compared to the left gland, grew significantly
as invasive cancers developed. Lymph nodes (LN) and tumors (Tu) are labeled. A
scale bar of 2 mm is also shown.
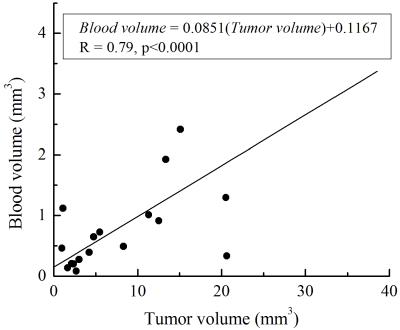
Figure 3. Plot of blood volume as a function of tumor volume in SV40Tag mice. The
scatter plot shows the relationship between the tumor and blood volumes measured
by MRI in a total of nine SV40 Tag mice of 18-20 weeks of age. There was a
strong positive correlation (r = 0.79, p < 0.0001) between the tumor volume
and blood volume as indicated in the plot.
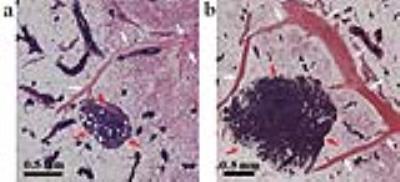
Figure 4. Histological images of mouse
mammary glands of a SV40TAg mouse. Hematoxylin and eosin (H&E)-stained
images of the excised a) left and b) right inguinal glands of a SV40Tag mouse
at 19 weeks of age are compared. Increased and more
dilated blood vessels associated with the larger tumor are seen on the right
inguinal gland (b) compared to the left gland (a). In both images tumors are
indicated by red arrows, while blood vessels are indicated by while arrows. A
scale bar of 0.5 mm in each image is also shown.