4480
Dietary Fat Results in Increased Tumor Burden in a Mouse Model of Human Triple-Negative Breast Cancer Based on Magnetic Resonance Imaging and Histology1Radiology, The University of Chicago, Chicago, IL, United States, 2Medicine, Sections of Adults and Pediatric Endocrinology, Diabetes and Metabolism, The University of Chicago, Chicago, IL, United States, 3Pathology, The University of Chicago, Chicago, IL, United States, 4Medicine, the Section of Hematology and Oncology, The University of Chicago, Chicago, IL, United States
Synopsis
Breast cancer is the most commonly diagnosed malignancy among women in the United States and the second leading cause of cancer mortality worldwide. Epidemiological studies suggest an increase in the risk of triple-negative breast cancer (TNBC) in association with a high animal fat diet. Based on previous MRI studies in SV40Tag mice, we examined the effect of pre-pubertal exposure to high dietary fat in this model of TNBC. The results reported here demonstrate that a high animal fat diet significantly increased the number of aggressive cancers detected by MRI in a mouse model of human TNBC.
PURPOSE
High dietary animal fat consumption is associated with an increase in triple negative breast cancer (TNBC) risk.1,2 Based on previous magnetic resonance imaging (MRI) studies demonstrating the feasibility of detecting very early non-palpable mammary cancers in SV40Tag mice,3 we examined the effect of pre-pubertal exposure to high dietary fat in this model of TNBC.METHODS
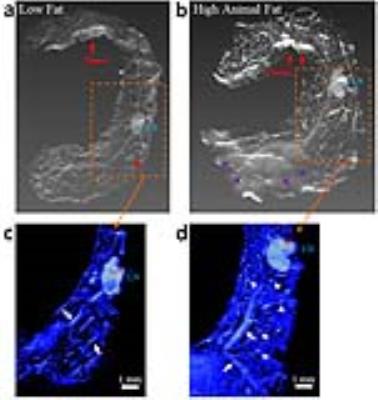
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and approved institutional approval. Virgin female C3(1)SV40TAg mice (n=16) were weaned at 3-4 weeks of age and then fed either a low fat diet (LFD) (n=8, 3.7 kcal/g; 17.2% kcal from vegetable oil) or a high animal fat diet (HAFD) (n=8, 5.3 kcal/g; 60% kcal from lard). After 8 weeks (12 weeks of age), fast spin echo MR images (RARE, TR/TEeffective=4000/20 ms, slice thickness=0.5 mm, and in-plane resolution of 0.1 mm) of inguinal mammary glands were acquired at 9.4T. Following in vivo MRI, mice were sacrificed and inguinal mammary glands were excised and formalin fixed for ex vivo MRI (3D-RARE, TR/TEeffective=4000/25 ms, isotropic resolution of 0.07 mm) and histology. 3D volume-rendered MR images were then correlated with mammary gland histology to assess both the glandular parenchyma and tumor burden. High resolution ex vivo MR images were used to evaluate ductal architecture and facilitate correlation of in vivo images with histology. Tumors were classified as in situ or invasive cancer based on MRI by observers who were blinded to histopathology. Student’s t-Tests were performed for statistical analysis. A p-value <0.05 was considered significant.RESULTS and DISCUSSION
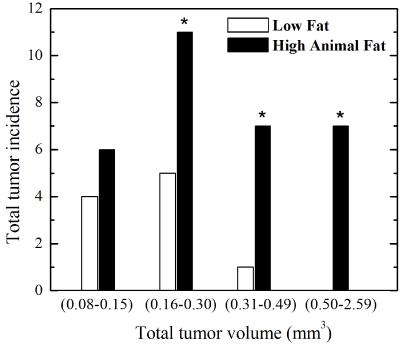
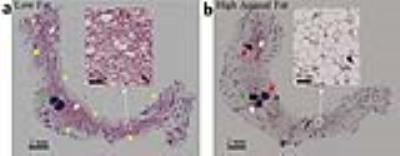
Mice given a HAFD did not gain significantly more weight than the LFD fed mice (average body weight of 18.20±1.04 g on LFD, compared to an average body weight of 19.75±2.02 g on HAFD, p<0.075). Based on the sizes of in situ (150 to 400 microns in largest diameter) and invasive cancers (>400 microns in largest diameter) and their signal intensities on T2-weighted MR images of 1.4 times that of muscle and of 2.3 times that of muscle, in situ cancers and invasive cancers, respectively, were accurately identified on in vivo MRI. Axial MR images of mammary glands of mice fed LFD (Figure 1a) versus HAFD (Figure 1b) showed fewer areas of DCIS and shorter T2 in the LFD mice. More DCIS, with longer T2, was found in HAFD mice, indicating shorter cancer latency. HAFD mice have thicker and more irregular ductal structure (Figure 1b, also shown in the ex vivo images of excised gland in Figure 2), suggesting more active mammary ducts as well as of abnormal ductal development. A plot (Figure 3) comparing the tumor incidence as a function of tumor volume in LFD and HAFD SV40TAg mice shows distinct differences between the two groups. The number of tumors with a volume of 0.08-0.15 mm3 was not significantly different in the LFD and HAFD groups. However, the difference between the two groups in the incidence of larger cancers was statistically significant (asterisks in Figure 3). An average of 3.88±1.03 tumors were detected per HAFD fed mouse compared to an average of 1.25±1.16 per mouse fed a LFD, (p<0.007). Average tumor volume was significantly higher in the HAFD group (0.53±0.45 mm3) compared to LFD group (0.20±0.08 mm3, p<0.02; see Table 1). Ex vivo MRI (Figure 2) and histology (Figure 4) both demonstrated that HAFD mice had denser parenchyma, more irregular and enlarged ducts, and dilated blood vessels, compared to LFD mice. MRI spectra were consistent with increased white adipose tissue (based on T2*), and this was confirmed by histology. Histology showed tumor invasion (Figure 4).CONCLUSIONS
SV40TAg mice in the HAFD did not significantly gain more weight compared to the mice on a regular diet, suggesting that critical changes affecting mammary gland anatomy and mammary cancer incidence occur in the mammary glands independently of systemic obesity. MRI and histological studies of the SV40TAg mice demonstrated that HAFD feeding from weaning through puberty resulted in a higher incidence and larger triple negative mammary tumors as well as altered mammary gland anatomy. Unlike other methods for assessing effects of environmental factors on mammary cancer, MRI allows routine serial functional and anatomic measurements, including detection of small intra-ductal cancers, accurate tumor volume measurements, and assessment of the 3-dimensional distribution of cancers over time. The present results are a first step towards routine use of an MRI ‘toolkit’ to assess effects of diet and dietary intervention on cancer risk and incidence in mouse models.Acknowledgements
This research is supported by grants from the National Institutes of Health (R01-CA133490 and R01-CA167785), Florsheim foundation, Segal foundation, and VPH prism grant from the European Union. The Lynn S. Florsheim Magnetic Resonance Laboratory subcore of the Integrated Small Animal Imaging Research Resource is partially supported by funds from the University of Chicago Comprehensive Cancer Center from the National Cancer Institute Cancer Center Support Grant P30CA014599.References
1. Yang XR, Chang-Claude J, Goode EL, et al. Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. J Natl Cancer Inst. 2011;103(3):250-263.
2. Agurs-Collins T, Dunn BK, Browne D, Johnson KA, Lubet R. Epidemiology of health disparities in relation to the biology of estrogen receptor-negative breast cancer. Semin Oncol. 2010;37(4):384-401.
3. Mustafi D, Zamora M, Fan X, Markiewicz E, Mueller J, Conzen SD, Karczmar GS. MRI accurately identifies early murine mammary cancers and reliably differentiates between in situ and invasive cancer: correlation of MRI with histology. NMR Biomed. 2015;28(9):1078-1086.
Figures