4479
Telomerase expression enhances pentose phosphate pathway flux resulting in an MR-detectable increase in reduced glutathione levels in mutant IDH1 glioma cells1Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States, 2Neurological Surgery, University of California San Francisco, San Francisco, CA, United States
Synopsis
Telomerase is the enzyme responsible for maintenance of telomeres, which are special capped structures that protect chromosomal ends from degradation. Telomerase activation and metabolic reprogramming have both emerged as hallmarks of cancer. Here, we investigated the link between telomerase and metabolism in mutant isocitrate dehydrogenase 1 (IDH1) glioma cells with the goal of identifying MR-detectable biomarkers of telomerase expression. Using 13C- and 1H- MRS, we show that telomerase expression is associated with elevated flux through the pentose phosphate pathway resulting in increased levels of reduced glutathione (GSH). Thus, GSH is a potential biomarker of telomerase expression in mutant IDH1 gliomas.
Introduction
Telomerase is the enzyme involved in the maintenance of telomeres, which are special capped structures that protect chromosomal ends from degradation1. Telomerase activation and metabolic reprogramming have both emerged as hallmarks of cancer2. In low-grade gliomas expressing the isocitrate dehydrogenase 1 (IDH1) mutation, telomerase expression is reactivated as part of the tumorigenic process3. Here, we investigated the link between telomerase and cellular metabolism with the ultimate goal of identifying metabolic biomarkers of telomerase expression in mutant IDH1 glioma cells. Using 13C-MRS we show that the fractional flux of [2-13C]-glucose to the pentose phosphate pathway (PPP) is higher in telomerase-expressing glioma cells relative to non-telomerase-expressing controls. We also show that levels of NADPH, a key product of the PPP, are higher in telomerase-expressing cells. One of the functions of NAPDH is to maintain glutathione in its reduced state4. Using 1H-MRS, we show that levels of reduced glutathione (GSH) are higher in telomerase-expressing glioma cells. Taken together, our study identifies increased GSH as an MR-detectable metabolic biomarker of telomerase expression in glioma cells.Methods
Immortalized normal human astrocytes without telomerase (NHApre) and with telomerase expression (NHApost and NHAhTERT) were generated as described3. All cell lines express the mutant IDH1 enzyme and produce 2-hydroxyglutarate. For 13C-MR studies, cells were cultured in medium containing 5mM [2-13C]-glucose for 96h (NHApre) or 48h (NHApost and NHAhTERT) followed by dual-phase extraction5. Proton-decoupled 13C-MR spectra (30°pulse, 3s relaxation delay, 2560 scans) were obtained on a 500-MHz Bruker spectrometer with a triple resonance cryoprobe. 1H-MR spectra were acquired using a 1D water presaturation ZGPR sequence, 90°pulse, 3s relaxation delay and 256 scans. Peak integrals were quantified, corrected for saturation effects and normalized to an external reference of known concentration (trimethylsilyl propionate). The fractional flux of glucose through the PPP was measured by taking advantage of the different isotopomers generated after tricarboxylic acid (TCA) cycle and PPP metabolism of [2-13C]-glucose as described6. Metabolism of three molecules of [2-13C]-glucose via the PPP leads to formation of two molecules of [4-13C]-glutamate. TCA cycle activity following glycolytic metabolism of 1 molecule of [2-13C]-glucose yields 1 molecule of [5-13C]-glutamate. PPP fractional flux can be therefore be calculated using the formula6
$$ \frac{\mbox{PPP}}{\mbox{PPP}+\mbox{TCA}} = \left( \frac{\frac{3}{2} * \left[ 4\mbox{-}^{13} \mbox{C} \right]\mbox{-glutamate}}{\frac{3}{2}* \left[4\mbox{-}^{13} \mbox{C} \right] \mbox{-glutamate} + \left[5\mbox{-}^{13} \mbox{C}\right] \mbox{-glutamate}} \right)$$
GSH/GSSG and NADPH/NADP+ ratio were determined spectrophotometrically using commercial kits (Abcam). Experiments were performed in triplicate (n=3) and statistical significance assessed using an unpaired t-test with p<0.05 considered significant.
Results
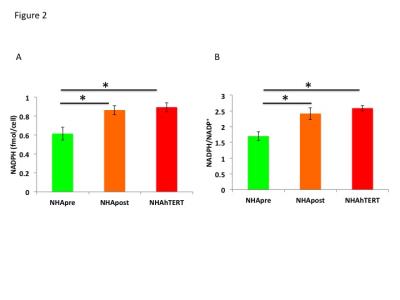
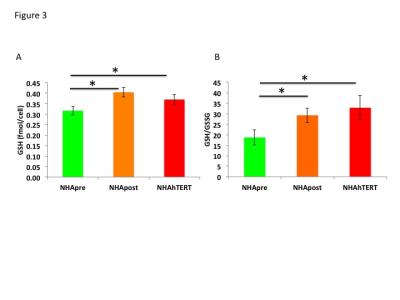
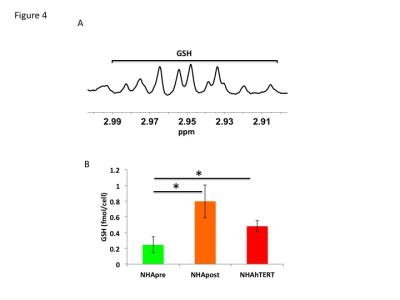
First, using western blotting, we confirmed that telomerase was expressed in NHApost and NHAhTERT but not NHApre cells (Fig.1A). We then labeled cells with [2-13C]-glucose to measure fractional flux through the PPP. A representative 13C-MR spectrum showing [4-13C]-glutamate (34.4ppm) derived from PPP activity and [5-13C]-glutamate (182.2ppm) resulting from TCA cycle activity is shown in Fig.1B. Quantification of 13C-glutamate levels showed that [4-13C]-glutamate levels increased by 72.1% (p<0.005) in NHApost cells and by 87.3% (p<0.001) in NHAhTERT cells compared to NHApre (0.05±0.02fmol/cell in NHApre to 0.17±0.02fmol/cell in NHApost and 0.37±0.02fmol/cell in NHAhTERT). Concomitantly, [5-13C]-glutamate levels decreased by 86% (p<0.001) in NHApost cells and by 33.8% (p<0.001) in NHAhTERT cells relative to NHApre (0.6±0.07fmol/cell in NHApre cells to 0.08±0.04fmol/cell in NHApost and 0.4±0.08fmol/cell in NHAhTERT). As a result, PPP fractional flux increased significantly from 10.4±5.4% in NHApre cells to 75.1±11.5% in NHApost cells and to 58.2±5.6% in NHAhTERT cells (Fig.1C). Next, we measured levels of NAPDH, a major product of the PPP, using a spectrophotometric assay. As shown in Fig.2A, NAPDH levels were significantly higher by 28.8% (p<0.01) in NHApost and by 31.2% (p<0.01) in NHAhTERT cells relative to NHApre cells. We also found a significant increase in the NAPDH/NADP+ ratio by 29.7% (p<0.01) in NHApost and by 34.4% (p<0.005) in NHAhTERT cells relative to NHApre cells (Fig.2B). We then measured GSH levels by spectrophotometry and found a significant increase by 21.8% (p<0.01) in NHApost and by 14.4% (p<0.05) in NHAhTERT cells relative to NHApre (Fig.3A). The GSH/GSSG ratio was also significant higher by 36.1% (p<0.05) in NHApost and 43.1% (p<0.05) in NHAhTERT cells relative to NHApre (Fig.3B). Finally, in order to assess the potential of GSH as an MR-detectable biomarker of telomerase expression, we measured GSH levels by 1H-MRS (Fig.4A). We found a significant increase in GSH levels by 69.4% (p<0.05) in NHApost and by 49.3% (p<0.05) in NHAhTERT relative to NHApre cells (Fig.4B).Conclusions
Collectively, our results indicate that telomerase expression induces an increase in PPP flux resulting in increased NAPDH and GSH levels in mutant IDH1 glioma cells. Increased GSH provides a unique metabolic biomarker of telomerase expression and potential therapeutic target in mutant IDH1 gliomas.Acknowledgements
Grant acknowledgements: NIH R01CA172845, NIH R01CA197254, NIH R01CA154915, NIH P41EB013598, UCSF Brain Tumor Loglio Collective and NICO.References
1) Mocelin et al., Trends in Molecular Medicine, 19(2): 125-133, 2013. 2) Hanahan & Weinberg, Cell, 144: 646-674, 2011. 3) Ohba et al., Cancer Research, canres.0696.2016, 2016. 4) Ying, Antioxidants & Redox Signaling, 10: 179-215, 2008. 5) Izquierdo-Garcia et al., PLOS ONE, 10:e0118781, 2015. 6) Delgado et al., Magnetic Resonance in Medicine 51:1283–1286, 2004.Figures