4472
Molecular effects of various chemotherapeutic agents on choline phospholipid metabolism of triple-negative breast cancer cells1Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
The MRS-detected total choline (tCho) signal is a promising non-invasive surrogate marker of chemotherapy response in breast cancer patients. The molecular mechanisms by which common chemotherapeutic drugs affect the tCho signal, consisting of glycerophosphocholine (GPC), phosphocholine (PC), and free choline (Cho), are unknown. We have employed widely used cancer chemotherapeutic drugs including doxorubicin, paclitaxel, and vinorelbine to treat triple-negative human MDA-MB-231 breast cancer cells to elucidate their molecular effects on choline phospholipid metabolism using high-resolution 1H MRS to detect changes in cellular choline metabolite profiles, and quantitative RT-PCR to assess the corresponding changes in the expression levels of choline-metabolizing enzymes.
Purpose
The magnetic resonance spectroscopy (MRS)-detected total choline (tCho) signal is a promising non-invasive surrogate marker of chemotherapy response in breast cancer patients [1-3]. However, the molecular mechanisms by which common chemotherapeutic drugs affect the tCho signal, which consists of glycerophosphocholine (GPC), phosphocholine (PC), and free choline (Cho), are mostly unknown. Here we have employed some widely used cancer chemotherapeutic drugs such as doxorubicin, paclitaxel, and vinorelbine to treat triple-negative human MDA-MB-231 breast cancer cells to elucidate their molecular effects on choline phospholipid metabolism. We have utilized high-resolution (HR) 1H MRS to detect changes in cellular choline metabolite profiles, and quantitative RT-PCR (qRT-PCR) to assess the corresponding changes in the expression levels of choline-metabolizing enzymes.Target Audience
Basic scientists interested in discovering metabolic biomarkers of drug treatment response and clinicians interested in non-invasive MRS methods to monitor therapeutic response in breast cancer.Methods
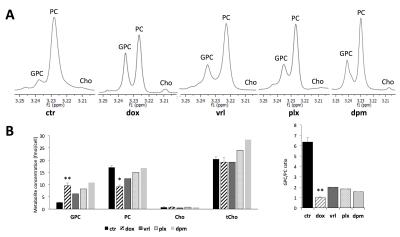
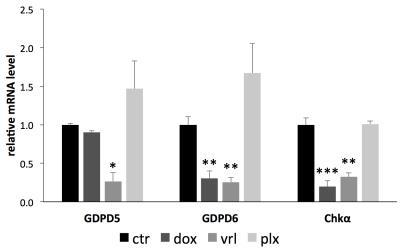
Triple-negative, highly aggressive MDA-MB-231 breast cancer cells were treated with 5 μM doxorubicin, 0.5 μM paclitaxel, 0.5 μM vinorelbine, 20 µM GDPD6 inhibitor dipyridamole, or DMSO vehicle control for 48 hours. Metabolites were extracted using dual-phase extraction (methonal:chloroform:water=1:1:1). High-resolution (HR) 1H MRS of the water-soluble extract fraction was performed on a Bruker 750 MHz MR spectrometer. Choline containing metabolites were quantified from MR spectra using MestReNova software. RNA extracted from cells with the same treatment was reverse transcribed, and SYBR Green based quantitative PCR was used to detect changes in mRNA levels of glycerophosphodiester phosphodiesterase domain containing 5 (GDPD5) [4] and 6 (GDPD6) [5], and choline kinase α (Chkα) [6].Results
After 48 hours of doxorubicin treatment, the GPC levels in MDA-MB-231 cells increased, while PC level decreased significantly, and the tCho concentration remained unchanged. Vinorelbine treatment displayed a comparable effect to doxorubicin, but the GPC increase and PC decrease occurred to a lesser extend. Paclitaxel treatment caused an increased GPC level along with an unaltered PC level, leading to a slightly increased tCho level. In all these cases, the PC/GPC ratio decreased, from 3.5 in the control to about 2.0 in paclitaxel- or vinorelbine-treated samples, or even about 1.0 in doxorubicin-treated samples. qRT-PCR-detected mRNA expression levels showed that GDPD6 and Chkα were downregulated by doxorubicin. GDPD5, GDPD6, and Chkα genes displayed a reduced mRNA expression level following vinorelbine treatment, while no significant change in these three genes was detected after paclitaxel treatment. For comparison, the known GDPD6 inhibitor dipyridamole significantly increased cellular GPC levels, but, as expected, did not affect GDPD6 mRNA expression levels as it acts as an enzyme inhibitor.Discussion
This study demonstrates that choline containing metabolites change differently depending on the type of drug used for the treatment of breast cancer. In the case of doxorubicin and vinorelbine, the tCho level did not change following anticancer treatment with these chemotherapeutic agents, whereas the individual components within the tCho signal, such as GPC and PC did change. In contrast, in the case of paclitaxel, the increased GPC and unaltered PC levels led to an increased tCho level. An increase in GPC with a concomitant decrease in PC is a change towards a choline metabolite profile that is typical of nonmalignant breast cancer cells [7]. This metabolic alteration away from the ‘cholinic phenotype’ was caused by doxorubicin- or vinorelbine-induced decreases in Chkα, GDPD6, and, in the case of vinorelbine, GDPD5 as well. Our study exemplifies that it is important to develop MRS methods, such as for example 31P MRS of the breast [8], that are able to detect GPC and PC individually for detecting the response to chemotherapy.Conclusions
The choline metabolite concentrations of GPC and PC and the PC/GPC ratio may serve as non-invasive surrogate makers of therapeutic response in triple-negative breast cancer patients undergoing chemotherapy with doxorubicin.Acknowledgements
No acknowledgement found.References
1. Bolan PJ. Magnetic resonance spectroscopy of the breast: current status. Magn Reson Imaging Clin N Am. 2013, 21(3):625-639.
2. Baek HM, Chen JH, Nie K, Yu HJ, Bahri S, Mehta RS, Nalcioglu O, Su MY. Predicting pathologic response to neoadjuvant chemotherapy in breast cancer by using MR imaging and quantitative 1H MR spectroscopy. Radiology. 2009, 251(3):653-662.
3. Meisamy S, Bolan PJ, Baker EH, Bliss RL, Gulbahce E, Everson LI, Nelson MT, Emory TH, Tuttle TM, Yee D, Garwood M. Neoadjuvant chemotherapy of locally advanced breast cancer: Predicting response with in vivo 1H MR spectroscopy pilot study at 4 T. Radiology. 2004, 233(2):424-431.
4. Cao MD, Dopkens M, Krishnamachary B, Vesuna F, Gadiya MM, Lonning PE, Bhujwalla ZM, Gribbestad IS, Glunde K. Glycerophosphodiester phosphodiesterase domain containing 5 (GDPD5) expression correlates with malignant choline phospholipid metabolite profiles in human breast cancer. NMR Biomed. 2012, 25(9):1033-1042.
5. Stewart JD, Marchan R, Lesjak MS, Lambert J, Hergenroeder R, Ellis JK, Lau CH, Keun HC, Schmitz G, Schiller J, Eibisch M, Hedberg C, Waldmann H, Lausch E, Tanner B, Sehouli J, Sagemueller J, Staude H, Steiner E, Hengstler JG. Choline-releasing glycerophosphodiesterase EDI3 drives tumor cell migration and metastasis. Proc Natl Acad Sci U S A. 2012, 109(21):8155-8160.
6. Glunde K, Jie C, Bhujwalla ZM. Molecular Causes of the Aberrant Choline Phospholipid Metabolism in Breast Cancer. Cancer Res. 2004, 64(12):4270-4276.
7. Aboagye EO, Bhujwalla ZM. Malignant transformation alters membrane choline phospholipid metabolism of human mammary epithelial cells. Cancer Res. 1999, 59(1):80-84.
8. Wijnen JP, van der Kemp WJ, Luttje MP, Korteweg MA, Luijten PR, Klomp DW. Quantitative 31P magnetic resonance spectroscopy of the human breast at 7 T. Magn Reson Med. 2012, 68(2):339-348.
Figures