4470
A Genetically Engineered Mouse Model Recapitulates Radiological Features of Human Adamantinomatous Craniopharyngioma1Division of Radiotherapy and Imaging, The Institute of Cancer Research, London, United Kingdom, 2Histopathology Department, Great Ormond Street Hospital for Children NHS Foundation Trust, London, United Kingdom, 3Birth Defects Research Centre, Institute of Child Health, University College London, London, United Kingdom, 4Division of Clinical Sciences, The Institute of Cancer Research, London, United Kingdom
Synopsis
Tumours in Hesx1Cre/+;Ctnnb1lox(ex3)/+ mice resemble human adamantinomatous craniopharyngioma (ACP) molecularly and histologically. MRI and ex vivo micro-CT were used to assess the radiology of this model for the first time. Early enlargement and heterogeneity of Hesx1Cre/+;Ctnnb1lox(ex3)/+ pituitaries was evident; enlargement of a solid tumour, and development of cysts and haemorrhage subsequently occurred. Solid components showed heterogeneous T1-weighted signal enhancement following Gd-DTPA administration, and in some animals cysts were hyperintense on FLAIR and T1-weighted images, both emulating clinical observations. Cyst calcification was not observed by micro-CT but we show that Hesx1Cre/+;Ctnnb1lox(ex3)/+ tumours faithfully recapitulate the MRI radiology of the human disease.
Introduction
Adamantinomatous craniopharyngiomas (ACPs) are histologically benign but clinically aggressive pituitary tumours mostly affecting children. ACPs present as mixed lesions with cystic and solid components, and are treated with surgery, radiotherapy and cystic drainage, but no specific chemotherapeutics currently exist. Mutations in CTNNB1, which encodes β-catenin, resulting in over-activation of the Wnt pathway are present in most human ACPs.1 Expression of oncogenic β-catenin in the developing pituitary of Hesx1Cre/+;Ctnnb1lox(ex3)/+ mice results in tumours resembling human ACP at the histological and molecular levels.2 However, the radiology of this model has not been evaluated.Methods
MRI was performed on Hesx1Cre/+;Ctnnb1lox(ex3)/+ mice (n=17) and control littermates (n=3) using a 7T Bruker microimaging system with a 3cm birdcage coil over a 2.5cm field-of-view. Multi-slice T2-weighted images (RARE; TR=4500ms, TEeff=36ms) were used to monitor tumour development alongside fluid attenuated inversion recovery (FLAIR (RARE; TR=18000ms, TEeff=35ms, TI=2100ms)) and T1-weighted (RARE; TR=1300ms, TEeff=7.5ms) imaging. At study end, echo-planar diffusion-weighted imaging (EPI-DWI; TR=1500ms, TEeff=32ms, 5 b-values; b=200-1000mm-2s) was used to determine the apparent diffusion coefficient (ADC) and T1-weighted images were acquired before and 1 minute after intravenous administration of 0.1mmolkg-1 Gd-DTPA (Magnevist, Schering) to assess the distribution of contrast enhancement. DWI data were fitted on a voxel-by-voxel basis using a Bayesian maximum a posteriori algorithm within in-house software, providing maps of spatial heterogeneity of ADC and median values for ROIs are reported.
Micro-CT images were acquired from formalin-fixed, iodinated (2.5% Lugol’s iodine for 72h) intact mouse heads using a Nikon XTH 225 ST micro-CT scanner, and data was processed and analysed in Volume Graphics® software and Imaris® (Bitplane), respectively. Tissue was then decalcified and paraffin-embedded and 5µm sections were subsequently stained with haematoxylin and eosin (H&E).
Results and Discussion
T2-weighted MRI performed from approximately 8 weeks of age demonstrated enlargement and increased heterogeneity of the pituitary in Hesx1Cre/+;Ctnnb1lox(ex3)/+ mice compared to controls. Abnormal pituitary architecture was also observed ex vivo by micro-CT (Figure 1). Presentation on T2-weighted images remained stable prior to the development of hyperintense cysts, enlargement of a solid portion of the tumour and presentation of hypointense regions found to be haemorrhagic/haematomas. Progression to these highly heterogeneous lesions was identified at a median age of 17.7 weeks (range 8.3-51.4 weeks, n=14). In some animals the cysts remained hyperintense on FLAIR and T1-weighted images (Figure 2, arrowed), which is observed clinically and attributed to high cystic protein and cholesterol levels.3 Other mice presented with cysts that attenuated on FLAIR to the same degree as ventricular cerebrospinal fluid and were isointense to the brain on T1-weighted images. Some lesions displayed both cystic phenotypes. Parametric ADC maps demonstrated that the solid tumour component had a similar ADC to the brain and that there was substantial heterogeneity corresponding to the different elements (Figure 2). Quantification showed that ADC was lowest in the solid component (635±29x10-6mm2s-1), was significantly higher in the haemorrhagic component (856±79x10-6mm2s-1, p=0.02, unpaired Student’s t-test) and was higher still in the cystic regions (1953±73x10-6mm2s-1, p<0.0001 vs solid and haemorrhagic). The cystic fluid evidently allows relatively free water diffusion, whereas diffusion in the haemorrhagic component was less restricted than in solid tumour but possessed more barriers to free diffusion than cysts, possibly due to the presence of clotting blood. Heterogeneous signal enhancement was observed on T1-weighted images following Gd-DTPA administration, which recapitulates the patterns of enhancement observed in ACP patients;3 no response was observed in the cystic or haemorrhagic components (Figure 2, lower panel).
Micro-CT performed on the heads of two mice that had undergone MRI immediately before sacrifice mirrored the heterogeneity observed on MRI (Figure 3). Whilst tissue shrinkage during fixation affected the integrity of large cysts, small cysts (open head arrows) and areas of haemorrhage (closed head arrows) within the tumour masses were evident on micro-CT images and H&E stained sections of the same tissue. The calcification of cystic fluid that is evident by CT in patients3 was not observed in the preclinical model.
The predominant cause of morbidity in ACP patients is infiltration of the hypothalamus and visual pathways, as well as destruction of the pituitary.4 In mice however, the tumours do not invade into the brain, likely as a result of differences in the hypothalamo-pituitary axis anatomy between mice and humans, with the predominant cause of morbidity being raised intracranial pressure due to haemorrhage and cystic volume.
Conclusion
The Hesx1Cre/+;Ctnnb1lox(ex3)/+ mouse model recapitulates a number of the key radiological features of childhood ACP and provides a promising foundation for imaging embedded in vivo trials of potential therapeutics for the treatment of the disease.Acknowledgements
We acknowledge the Cancer Research UK support to the Cancer Imaging Centre at The Institute of Cancer Research and The Royal Marsden Hospital in association with the MRC and Department of Health (England) (C1060/A16464), a Cancer Research UK Fellowship Grant, NHS funding to the NIHR Biomedical Research Centre at The Royal Marsden and the ICR, and Great Ormond Street Hospital Biomedical Research Centre, the MRC (MR/M125/1) and Children with Cancer UK (15/190).References
1. Buslei R, Nolde M, Hofmann B, et al. Common mutations of beta-catenin in adamantinomatous craniopharyngiomas but not in other tumours originating from the sellar region. Acta Neuropathol. 2005;109:589-597.
2. Gaston-Massuet C, Andoniadou CL, Signore M, et al. Increased Wingless (Wnt) signaling in pituitary progenitor/stem cells gives rise to pituitary tumors in mice and humans. Proc Natl Acad Sci U S A. 2011;108:11482-11487.
3. Curran JG, O'Connor E. Imaging of craniopharyngioma. Childs Nerv Syst. 2005;21:635-639.
4. Muller HL. Childhood craniopharyngioma--current concepts in diagnosis, therapy and follow-up. Nat Rev Endocrinol. 2010;6(11):609-18.
Figures
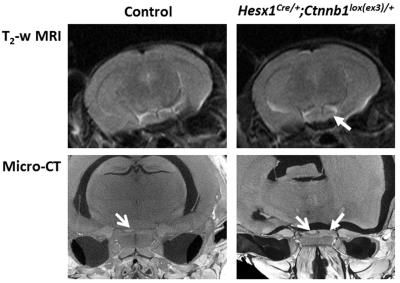
Figure 1.
Axial in vivo T2-weighted MRI (upper panel) and ex vivo micro-CT (lower panel) images of the pituitary region of 8 week old control and Hesx1Cre/+;Ctnnb1lox(ex3)/+ mice. Closed head arrows denote expansion and increased heterogeneity of the pituitaries of Hesx1Cre/+;Ctnnb1lox(ex3)/+ mice. Open head arrows denote the posterior lobe of the pituitary detectable on micro-CT images. MRI resolution 98x98x1000µm; micro-CT resolution ~9.1µm3.
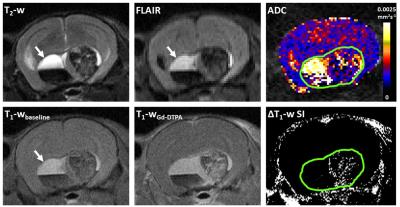
Figure 2.
Upper panel: T2-weighted and fluid attenuated inversion recovery (FLAIR) images, and a parametric map of apparent diffusion coefficient (ADC) acquired from a 1mm thick axial slice through a Hesx1Cre/+;Ctnnb1lox(ex3)/+ tumour bearing mouse head. Lower panel: Matched T1-weighted images acquired at baseline and 1 minute after injection of 0.1mmolkg-1 Gd-DTPA and a subtraction map clearly showing areas of signal enhancement. Green ROI denotes lesion volume. Arrows denote cystic region that did not attenuate on FLAIR and was hyperintense on T2-weighted and baseline T1-weighted MRI.
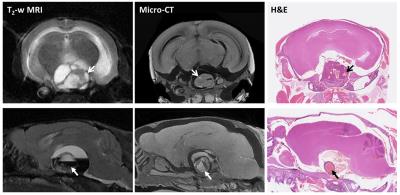
Figure 3.
In vivo T2-weighted MRI, ex vivo micro-CT and H&E stained FFPE sections from two Hesx1Cre/+;Ctnnb1lox(ex3)/+ mice. Open and closed head arrows denote cysts and haemorrhage within tumour masses, respectively. Slice thickness: MRI = 1000µm, micro-CT ≈ 9.1µm, tissue section = 5µm.