4467
Intrinsic susceptibility MRI predicts response to the vascular endothelial growth factor receptor inhibitor cediranib in the Th-MYCN model of neuroblastoma1Division of Radiotherapy and Imaging, The Institute of Cancer Research, London, London, United Kingdom, 2Division of Cancer Therapeutics and Division of Clinical Studies, The Institute of Cancer Research, London, London, United Kingdom
Synopsis
In this study we demonstrate that the transverse relaxation rate R2* predicts response to the vascular endothelial growth factor receptor (VEGFR) inhibitor cediranib in the Th-MYCN genetically-engineered murine model of neuroblastoma, a childhood cancer of the developing nervous system.
Introduction / Purpose
The clinical outcome of children with high-risk neuroblastoma, a cancer of the developing sympathetic nervous system, remains low. All neuroblastoma survivors have a high-risk of developing long-term disabilities and life-threatening conditions caused by current treatments. Better and safer therapies are urgently needed.1 In neuroblastoma, amplification of the proto-oncogene MYCN correlates with enhanced angiogenesis and poor survival. The hyper-vascular nature of high-risk neuroblastoma has prompted the evaluation of numerous small molecule inhibitors of the VEGF signaling pathway in early-phase pediatric clinical trials.2 Conventional pharmacodynamic biomarkers necessitate access to post therapy biopsies, which is often not practical in the pediatric population. Therefore, non-invasive alternatives, such as imaging biomarkers are being pursued. The Th-MYCN transgenic model of neuroblastoma faithfully recapitulates the major genetic, patho-physiological and radiologic features of the childhood disease, providing an information-rich platform to evaluate novel therapies and associated non-invasive biomarkers.3,4 We have previously demonstrated that cediranib is a potent inhibitor of VEGFR signaling in the Th-MYCN model, resulting in a rapid reduction in tumor perfusion 24h after treatment.3 In this study we demonstrate that the native transverse relaxation rate R2* can predict long-term tumor volume response to treatment with cediranib in the Th-MYCN model.Methods
All experiments were performed in accordance with the UK Home Office Animals (Scientific Procedures) Act 1986 and the United Kingdom National Cancer Research Institute guidelines for the welfare of animals in cancer research.5
Th-MYCN mice with spontaneously arising abdominal neuroblastoma were identified by palpation. On day 1, mice were treated daily with 6mg/kg cediranib (AstraZeneca) p.o. (n=36) or vehicle (n=12). Anatomical MRI was performed on day 0 and day 7. Native R2* was measured on day 0.
MRI studies were performed on a 7T Bruker MicroImaging system using a 3cm birdcage volume coil. T2-weighted axial images were used for determining tumor volumes and, following optimization of the local field homogeneity (Fastmap), to plan the R2* measurement (multi-gradient echo sequence, FOV 3x3cm2, 128x128 matrix, TE=6-30ms, 8 TEs, TR=200ms, NEX=8) as previously described.6
Results
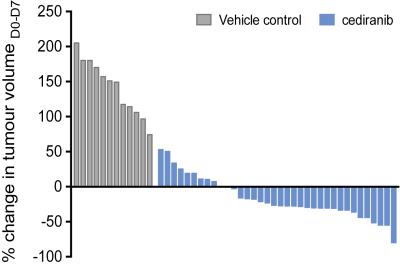
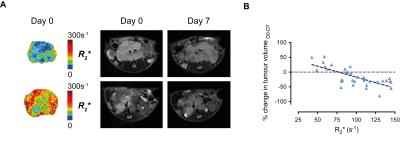
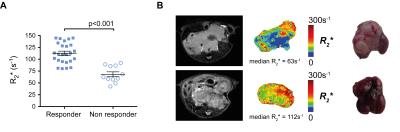
Cediranib significantly affected the characteristic aggressive tumor growth of the Th-MYCN model of neuroblastoma, yet with a spectrum of responses ranging from progressive disease to partial response (Fig 1.). Retrospective examination of the relaxometry data revealed that responsive tumors exhibited a characteristically fast native R2* pre-treatment (Fig 2.A.), whereas progressive tumors initially showed a slower native R2*. Cediranib-induced changes in tumor volume over 7 days treatment correlated with native tumor R2* before treatment (Fig 2.B. and 3.A.). Screening of the Th-MYCN mice colony before trial enrollment identified mice bearing tumors with relatively slow median R2* (<70s-1), which on necroscopy revealed very pale tumors, contrasting with the more characteristically dark red appearance of tumors in the majority of Th-MYCN mice (Fig 3.B.).Discussion
This study demonstrates that R2* is a potential predictive biomarker of response to cediranib in the Th-MYCN model of neuroblastoma. R2* quantification is rapid and completely non-invasive, and based on our previous work6, has already been incorporated routinely into early phase MRI-embedded clinical trials of novel therapies for children with neuroblastoma at the Royal Marsden Hospital (UK). VEGF expression is regulated at a transcriptional level via a MYCN-dependent mechanism, which explains the characteristic hypervascular and hemorrhagic phenotype of our MYCN-driven model of neuroblastoma.7 The high dependence of MYC-addicted neuroendocrine tumors on VEGF-driven angiogenesis was proposed as an explanation for the extensive wave of apoptosis and subsequent rapid tumor regression observed when MYC signaling is therapeutically switch off.8 We show that the extent of cediranib-mediated tumor debulking correlates with relatively fast native R2* and hence a high degree of hemorrhage/vascular instability in these tumors, illustrating a similar reliance on VEGF-signalling in our MYCN-addicted model of neuroblastoma. Phenotypic and molecular characterisation of the response of tumors with slow baseline R2* to cediranib is ongoing.Conclusion
Fast native tumor R2* is associated with response to the potent VEGFR inhibitor cediranib in the Th-MYCN model of neuroblastoma. Native tumor R2* measurement is a potential predictor biomarker in this disease and treatment setting.Acknowledgements
We acknowledge support from The Institute of Cancer Research Cancer Research UK and EPSRC Cancer Imaging Centre, in association with the MRC and Department of Health (England) grant C1060/A10334, NHS funding to the NIHR Biomedical Research Centre, a Cancer Research UK Programme Grant (C18339), a Children with Cancer UK project grant and a Children with Cancer UK Research Fellowship.References
1 Barone, G., Anderson, J., Pearson, A. D. et al. New strategies in neuroblastoma: Therapeutic targeting of MYCN and ALK. Clin Cancer Res. 2013; 19(21): 5814-5821.
2 Roy Choudhury, S., Karmakar, S., Banik, N. L. et al. Targeting Angiogenesis for Controlling Neuroblastoma. Journal of Oncology. 2012; 2012(15.
3 Chesler, L. & Weiss, W. A. Genetically engineered murine models--contribution to our understanding of the genetics, molecular pathology and therapeutic targeting of neuroblastoma. Semin Cancer Biol. 2011; 21(4): 245-255.
4 Jamin, Y., Tucker, E. R., Poon, E. et al. Evaluation of clinically translatable MR imaging biomarkers of therapeutic response in the TH-MYCN transgenic mouse model of neuroblastoma. Radiology. 2013; 266(1): 130-140.
5 Workman, P., Aboagye, E., Balkwill, F. et al. Guidelines for the welfare and use of animals in cancer research. Br J Cancer. 2010; 102(11): 1555-1577.
6 Jamin, Y., Glass, L., Hallsworth, A. et al. Intrinsic susceptibility MRI identifies tumors with ALKF1174L mutation in genetically-engineered murine models of high-risk neuroblastoma. PLoS One. 2014; 9(3): e92886.
7 Chanthery, Y. H., Gustafson, W. C., Itsara, M. et al. Paracrine Signaling Through MYCN Enhances Tumor-Vascular Interactions in Neuroblastoma. Science Translational Medicine. 2012; 4(115)
8 von Eyss, B. & Eilers, M. Addicted to Myc-but why? Genes & Development. 2011; 25(9): 895-897.
Figures