4456
USPIOs for metastatic lymph node detection in prostate cancer: back on the block1Radiology and Nuclear Medicine, Radboud university medical center, Nijmegen, Netherlands, 2Pharmacology, Radboud university medical center, Nijmegen, Netherlands
Synopsis
The presence of nodal metastases in patients with prostate cancer is a key factor determining prognosis and treatment. With the reintroduction of ferumoxtran-10-enhanced MRI in local clinical practice, small metastases in lymph nodes down to 2 mm in size can be detected non-invasively, avoiding diagnostic surgical lymph node dissections and guiding personalized treatment of patients with prostate cancer at intermediate and high risk for metastatic spread.
Clinical Question
Is it possible to non-invasively diagnose the presence of small lymph node metastases in patients with prostate cancer?Impact
Prostate cancer is the most prevalent non-cutaneous cancer in men. With improved detection and assessment of aggressiveness of localized disease, the next question in disease management is one of the current most pressing issues for the individual patient: an accurate assessment of the nodal status for metastases. An early as possible detection of transformation from local to systemic disease is crucial for the treatment plan: localized disease can be cured with local surgery, but metastatic disease will need more extensive treatment and if guided properly, possibly with curative intent.Approach
Clinically, diagnostic (extended) surgical lymph node dissections are performed to assess the lymph node status in patients with prostate cancer with intermediate/high risk for metastases[1]. Histopathology of excised nodes establishes the nodal stage diagnosis. These surgical staging-procedures are associated with considerable (co-)morbidity, they can miss small positive nodes within the resection field, and will miss any positive nodes outside the resection field[2,3]. For node negative patients, removal of – in retrospect healthy – nodes is of no benefit to the patient. Magnetic Resonance Lymphography (MRL) with Combidex (i.e. ferumoxtran-10) is non-invasive diagnostic MRI examination[4]: a solution of nanoparticles of iron oxide with a sugar coating is slowly intravenously administered in patients, the nanoparticles are internalized by macrophages and accumulate inside normally functioning lymph nodes. Twenty-four to thirty-six hours after administration, the presence or absence of iron oxide in lymph nodes is the basis for contrast between healthy (no signal) and metastatic (bright signal) nodes on iron-sensitive T2*-weigthed MR examinations. Four years after ferumoxtran-10 was withdrawn from the registration process in Europe by the original manufacturer, a Dutch academic hospital obtained all rights and documents regarding Combidex, and has since 2014 produced and used the agent as a legitimate exception of National and EU legislation. Since the reintroduction as nano-MRI, 310 men with prostate cancer have been examined in this institute. At 3T, the two main pulse sequences used are an isotropic 3D T1-weighted gradient echo sequence (TR/TE/flip angle of 6.47ms, 2.46ms and 10° at 0.85x0.85x0.85mm resolution, acquisition time 8:03 min) and a T2*-weighted spoiled gradient echo sequence (at the same position and resolution as the T1w series with water selective excitation, TR/TE/flip angle of 21.0ms, 12.0ms (two combined echoes) and 10°, acquisition time 10:10 min). The water-selective iron-sensitive scan provides the desired contrast: blood vessels (still containing ferumoxtran-10) and metastatic lymph nodes appear white, lipid tissue and healthy nodes are black. The radiologist’s job is to distinguish metastatic lymph nodes from blood vessels by continuously observing all three dimensions of vessels and nodes, aided by the T1-weighted image series (in which nodes appear as black dots within lipid tissue).Gains and Losses
Replacing diagnostic surgery with nano-MRI is a major gain. Not only the morbidity and cost of surgery is prevented, nano-MRI covers the entire pelvis and upper abdomen, and identifies positive lymph nodes outside the routine surgical field. If nodal metastases are present, treatment can be adjusted to either treat them with curative intent (e.g. stereotactic radiotherapy) or – in case of extended disease – to choose for the palliative track. Without metastatic nodes, no surgical morbidity exists and the local prostate can be treated curatively (e.g. radical prostatectomy). Regarding the contrast agent, no severe reactions and seven contrast related minor adverse effects were observed after administration of ferumoxtran-10 in 310 patients (back pain, nausea and dry mouth). Currently, SPL Medical B.V. (Nijmegen, The Netherlands) is the only rights owner of ferumoxtran-10, and produces ferumoxtran-10 under GMP conditions as a lyophilisate formula and works on registration studies for the agent.
Reading nano-MRI images has a learning curve in recognizing small metastatic nodes from blood vessels, and especially ganglia can appear similarly as metastatic nodes. With proper training and experience, very high sensitivities and specificities for detecting metastatic nodes can be achieved[5]. As the concept is generic, nano-MRI can also be used in other cancers that metastasize to lymph nodes.
Preliminary Data
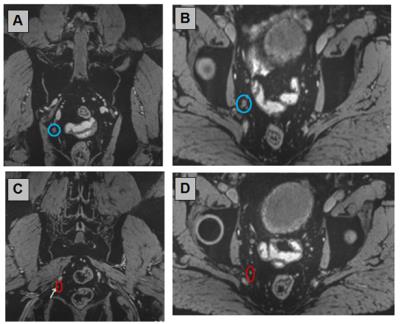
Without an alternate contrast agent or medical device for in vivo detection of small metastatic lymph nodes in prostate cancer (example in figure 1), the clinical patients rely on this technique for the N-stage of their disease and have their treatment plans arranged accordingly. Therefore no alternative diagnostic modality is used for these patients. The 10 provided examples illustrate the detection of metastases in small lymph nodes in different areas of the abdomen, illustrated with the T2*-weighted scans reconstructed in three orthogonal orientations.Acknowledgements
No acknowledgement found.References
1. Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T, Zattoni F, et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol 2014, 65:124-137.
2. Heesakkers RA, Jager GJ, Hovels AM, de Hoop B, van den Bosch HC, Raat F, Witjes JA, Mulders PF, van der Kaa CH, Barentsz JO. Prostate cancer: detection of lymph node metastases outside the routine surgical area with ferumoxtran-10-enhanced MR imaging. Radiology 2009, 251:408-414.
3. Meijer HJ, Fortuin AS, van Lin EN, Debats OA, Alfred Witjes J, Kaanders JH, Barentsz JO. Geographical distribution of lymph node metastases on MR lymphography in prostate cancer patients. Radiotherapy and Oncology 2013;106(1): pp.59-63.
4. Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH, de la Rosette J, Weissleder R. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med 2003, 348:2491-2499.
5. Heesakkers RA, Hovels AM, Jager GJ, van den Bosch HC, Witjes JA, Raat HP, Severens JL, Adang EM, van der Kaa CH, Futterer JJ, et al. MRI with a lymph-node-specific contrast agent as an alternative to CT scan and lymph-node dissection in patients with prostate cancer: a prospective multicohort study. Lancet Oncol 2008, 9:850-856.
Figures