4453
Diagnostic value of using SSFP and UTE subtraction MRI for the management of critical limb ischemia patients1Schulich Heart Program, Sunnybrook Research Institute, Toronto, ON, Canada, 2Division of Vascular Surgery, University of Toronto, Toronto, ON, Canada, 3Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada
Synopsis
There are two treatment options for revascularizing patients with critical limb ischemia: bypass surgery and percutaneous vascular intervention (PVI). PVI is less invasive but has high immediate technical failure rates (20%) and high re-intervention rates (20%). The most common mode of immediate failure is the inability to enter/cross the lesion. With current imaging (X-ray angiography, CTA, Duplex ultrasound) it is difficult to predict which lesions will be soft enough to cross with a wire to make PVI possible. Physicians have responded with a “percutaneous-first” strategy where they attempt PVI in all patients and perform surgery if PVI fails. This requires more procedures per index limb at significant cost to healthcare systems and delays definitive revascularization. Additionally, there is evidence that surgical bypass after failed PVI results in worse outcomes, including higher amputation rates within 1 year.
These issues highlight the critical need for improved diagnostic accuracy to inform patient selection. We have developed and validated MR methods to distinguish hard PAD lesions (densely calcified or collagenous) that would be at high risk of PVI failure from soft lesions that would be amenable to PVI. The impact of this work will help to reduce PVI failure rates, reduce time to definitive revascularization and reduce costs for additional procedures and investigations.
Clinical Question
When should critical limb ischemia patients be treated with percutaneous vascular intervention (PVI) instead of surgical bypass or amputation?Impact
Peripheral arterial disease (PAD) is a common, chronic problem. 10 million individuals in the US alone have the most severe form of PAD (critical limb ischemia), with 40% requiring a major amputation and up to 20% dying within just one year of diagnosis1.
There are two treatment options: bypass surgery and PVI. PVI is less invasive but PVI has high immediate technical failure rates (20%)2 and high re-intervention rates (20%)3. The most common mode of immediate failure is the inability to enter/cross the lesion. With current imaging (X-ray angiography, CTA, Duplex ultrasound) it is difficult to predict which lesions will be soft enough to cross with a wire to make PVI possible. Physicians have responded with a “percutaneous-first” strategy where they attempt PVI in all patients and perform surgery if PVI fails. This requires more procedures per index limb at significant cost to healthcare systems and delays definitive revascularization. Additionally, there is evidence that surgical bypass after failed PVI results in worse outcomes, including higher amputation rates within 1 year4–7. These issues highlight the critical need for improved diagnostic accuracy to inform patient selection.
We have developed and validated MR methods to distinguish hard PAD lesions (densely calcified or collagenous) that would be at high risk of PVI failure from soft lesions that would be amenable to PVI. The impact of this work will help to reduce PVI failure rates, reduce time to definitive revascularization and reduce costs for additional procedures and investigations.
Approach
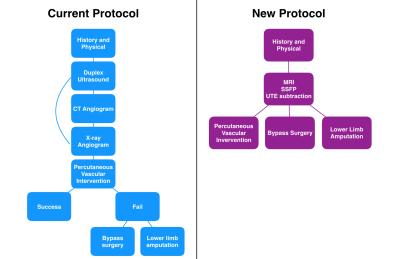
High value MRI protocol:
1) History and physical assessment including ankle brachial indices (ABI)
2) MRI study:
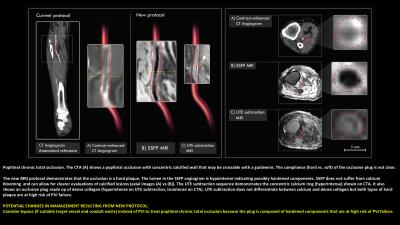
a. SSFP angiogram: A flow-independent scan that highlights blood based on relaxation properties and does not require exogenous contrast agents, so is ideal for imaging slow-flowing occlusive run-off arteries. This scan can be used to identify stenoses/occlusions and to assess tibial run-off vessels.
b. MR plaque characterization with ultrashort echo time (UTE) acquisitions, combined with image subtraction. UTE subtraction highlights short T2 plaque components including calcium and dense-collagen. These plaque components require high guidewire puncture forces that are not achievable with clinical guidewires, and therefore would be at high risk of immediate technical failure8. Based on this plaque characterization, patients will be referred for PVI if the target lesion is soft and likely crossable, bypass surgery if the lesion is hard and there is a suitable conduit and outflow vessel to bypass to, or amputation if the patient is at high likelihood of endovascular failure and has no outflow vessels for bypass.
Traditional approach:
1) History/Physical
2) Duplex ultrasound to evaluate location/length of lesions and degree of stenosis with blood flow velocities and Doppler wave forms.
3) CT Angiogram to assess femoropopliteal disease.
4) Invasive x-ray angiogram (current gold standard) to assess tibial vessel outflow.
5) Percutaneous-first approach – all patients undergo attempted PVI at the time of their X-ray angiogram. If unsuccessful, patients go on to surgical bypass if a suitable outflow vessel is identified or lower-limb amputation if there are no outflow vessels for bypass.
Gains and Losses
Gains: Our protocol will characterize plaque features including: degree of stenosis, calcification/collagen content, hard vs soft plaque components, eccentricity, and stump morphology with better accuracy. It also provides better visualization of vascular tree and microchannels (3D, flow-independent). Additional gains include: fewer tests/procedures, native contrast, and it is non-invasive.
Losses: Our protocol is more sensitive to motion, currently has poorer resolution compared to CTA and X-ray angiography, patients may have contraindications and there is no hemodynamic flow information (future protocols can incorporate phase-contrast MRA).
Preliminary Data
Our new protocol improves resource utilization because it decreases number of investigations, decreases interpretation time for physicians, decreases time to definitive revascularization, and avoids percutaneous attempts that may compromise future revascularization treatments.
This protocol may also help to improve PVI device/wire selection (Examples 1 & 2) and surgical bypass planning (Examples 6, 8, 9) to improve treatment outcomes. Ultimately our goal is to help improve limb salvage rates by identifying revascularization strategies for patients that are currently undergoing amputations (Examples 4, 5, 7, 9, 10). A recent cost-utility analysis showed that the 5-year costs of primary amputation was $152,426 versus PVI/surgery with repeated interventions as necessary ($121,478 - $124,696)9. For the entire US healthcare system, amputations cost $8.3 billion dollars annually. With informed treatment decision-making, it will be possible to improve treatments and limb salvage rates in this growing patient population.
Acknowledgements
We thank GE Healthcare for providing the UTE prototype sequence. This work was supported by the Canadian Institutes of Health Research (MOP-126169), Vanier Canada Graduate Scholarships program, the Blair Foundation and the Maggisano Research Chair in Vascular Surgery.References
1. Jessica P S, Schanzer A. Lower Extremity Arterial Disease: Decision Making and Medical Treatment. In: Cronenwett JL, Johnston KW, eds. Rutherford’s Vascular Surgery. 8th ed. Elsevier; 2014:1675-1700. doi:10.1016/B978-1-4160-5223-4.00104-9.
2. Bradbury AW, Adam DJ, Beard JD, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): Multicentre, randomised controlled trial. Lancet. 2005;366(9501):1925-1934. doi:10.1016/S0140-6736(05)67704-5.
3. Meltzer AJ, Sedrakyan A, Isaacs A, Connolly PH. Comparative effectiveness of peripheral vascular intervention versus surgical bypass for critical limb ischemia in the Vascular Study Group of Greater New York. J Vasc Surg. 2013;64(5):1320-1326.e2. doi:10.1016/j.jvs.2016.02.069.
4. Nolan BW, De Martino RR, Stone DH, et al. Prior failed ipsilateral percutaneous endovascular intervention in patients with critical limb ischemia predicts poor outcome after lower extremity bypass. J Vasc Surg. 2011;54(3):730-736. doi:10.1016/j.jvs.2011.03.236.
5. Ryer EJ, Trocciola SM, Derubertis B, et al. Analysis of outcomes following failed endovascular treatment of chronic limb ischemia. Ann Vasc Surg. 2006;20(4):440-446. doi:10.1007/s10016-006-9101-4.
6. Jones DW, Schanzer A, Zhao Y, et al. Growing impact of restenosis on the surgical treatment of peripheral arterial disease. J Am Heart Assoc. 2013;2(6):e000345. doi:10.1161/JAHA.113.000345.
7. Roy T, Forbes T, Wright G, Dueck A. Burning Bridges?: Mechanisms and Implications of Endovascular Failure in the Treatment of Peripheral Artery Disease. J Endovasc Ther. 2015;22(6):874-880. doi:10.1177/1526602815604465.
8. Roy T, Liu G, Shaikh N, Dueck AD, Wright GA. Puncturing Plaques?: Relating MRI Characteristics of Peripheral Artery Lesions to Guidewire Puncture Forces. J Endovasc Ther. doi:10.1177/1526602816671135.
9. Barshes NR,
Kougias P, Ozaki CK, et al. Cost-effectiveness of revascularization for limb
preservation in patients with marginal functional status. Ann Vasc Surg.
2014;28(1):10-17. doi:10.1016/j.avsg.2013.08.004.