4445
A Novel Cryogenic Radio-Frequency Probe for High Spatial Resolution Fluorine-19 MRI of Brain Inflammation1Berlin Ultrahigh Field Facility (B.U.F.F.), Max Delbrueck Center for Molecular Medicine, Berlin, Germany, 2Bruker BioSpin AG, Fällanden, Switzerland, 3Bruker BioSpin MRI GmbH, Ettlingen, Germany, 4MRI TOOLs GmbH, Berlin, Germany, 5Experimental and Clinical Research Center (ECRC), a joint cooperation between the Charité Medical Faculty and the Max Delbrueck Center, Berlin, Germany
Synopsis
MRI using i.v. administered fluorine-19 loaded nanoparticles (NPs) allows the tracking of inflammatory cell migration. The inherently low SNR limits the precise localization of 19F-labeled inflammatory cells, because large voxel sizes are needed to collect sufficient signal. To overcome this, we show here the first use of a novel 19F cryogenic quadrature RF surface probe at ultrahigh field to substantially boost SNR beyond that of state-of-the-art room temperature RF coils, while facilitating the acquisition of better spatially-resolved images within shorter scan times.
Purpose
Neuroinflammatory autoimmune diseases such as multiple sclerosis involve an early recruitment of inflammatory immune cells from the periphery into the central nervous system [1]. Previously we studied immune cell infiltration into brains of mice with experimental autoimmune encephalomyelitis (EAE), the animal model of MS, using fluorine-19 (19F)-loaded nanoparticles (NPs). Nanoparticles are administered intravenously (i.v.), and are taken up by inflammatory cells during their migration from the systemic circulation into the brain [2]. We detected the 19F-labeled cells in vivo using a room temperature (RT) dual 19F and 1H radio frequency (RF) volume resonator employing 3D imaging with isotropic spatial resolutions above 600µm to overcome 19F signal-to-noise ratio (SNR) constraints [2]. This limited the information on the precise location of 19F-labeled inflammatory cells in the brain. To overcome this, we show here the first use of a new 19F transceive cryogenic quadrature RF surface probe (CRP) at ultrahigh field to substantially boost SNR beyond that of standard state-of-the-art RT coils, while facilitating the acquisition of better spatially-resolved images within shorter scan times.Methods
Experiments were performed on a 9.4T animal MR system (Bruker BioSpin, Germany). The transceive quadrature CRP tuned to 376 MHz was compared with the volume resonator (ID=16mm) used previously [2]. SNR gain estimation: Using a 15ml-phantom filled with 30% 2,2,2-trifluoroethanol (TFE) in water, we acquired an axial 2D-RARE image (TR=3000ms, TE=42ms, ETL=8, FOV=20mm, matrix=96, NEX=8). B1-mapping: On the same phantom we acquired 3D-SE images (TR=6000ms, TE=4.5ms, FOV=28x16x16mm3, matrix=91x52x52, NEX=1) with nominal excitation flip angles (FA) of 60 and 120 degrees and calculated the actual FA using the double-angle method. SNR vs number of 19F atoms: NMR tubes (ID=4mm) were filled with different concentrations of 19F-loaded NPs (Z-average diameter=164nm), which were prepared using perfluoro-15-crown-5-ether (PFCE, Fluorochem, UK) [3]. Each tube was placed below the CRP surface leaving a 1mm gap. SNR was calculated in an axial 2D-RARE image (TR=3000ms, TE=21ms, ETL=8, FOV=20x20mm2, matrix=96x96, NEX=1). High-resolution brain image: Animal experiments were carried out in accordance with the local Animal Welfare Department. To induce EAE, SJL/J mice were immunized with proteolipid protein peptide139-151, and assessed daily for neurological symptoms [4]. PFCE NPs (10µmol) were administrated daily to EAE mice i.v. (D5-D9 following immunization). Mice were sacrificed on D10, fixed in paraformaldehyde and prepared in 15-ml tubes for ex-vivo MRI. 3D-RARE protocols were used for 19F MRI (TR=800ms, TE=4.9ms, FOV=28x16x16mm3, matrix=182x52x52, ETL=26, NEX=384) and a 1H reference image (TR=1300ms, TE=39ms, FOV=30x20x20mm3, matrix=192x128x128, ETL=16, NEX=3).Results
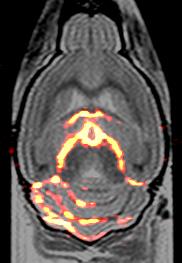
We first compared the performance of the CRP with the RT coil by estimating SNR of TFE phantom images acquired with both coils. The 19F CRP SNR gain was calculated by dividing the SNR for several ROIs (at increasing distances to the CRP surface) by the SNR of a large central ROI in the RT coil image (Fig. 1). Up to a depth of 8.5 mm the CRP had a superior SNR to the RT coil. Peak SNR gain exceeded a factor of 6. To investigate the SNR achieved as a function of the number of 19F atoms for a fast 2D-RARE protocol, we performed several scans with the 19F CRP using phantoms with varying concentrations of PFCE NPs (25mM-200mM) and with varying slice thickness (400µm-2000µm). The smallest number of 19F atoms per voxel measured (5.2×1015) resulted in an SNR of 18 (Fig. 2). B1-Mapping (Fig. 3) demonstrated the typical inhomogeneity of a surface coil array, very similar to those reported for 1H cryoprobes [5]. The significant flip angle variation must be compensated for by B1-correction in order to allow quantitative 19F imaging (Fig. 3). Using a 3D-RARE protocol as previously described [2], we next acquired 19F MR images of a fixed EAE mouse brain using the CRP. In these measurements we increased the spatial resolution from 400x400x400µm to 154x308x308µm. Preliminary results with this newly designed 19F CRP show a superior delineation of the areas of immune cell infiltration, especially within the myelinated regions of the cerebellum (Fig. 4) when compared to previous images obtained with the RT 19F volume resonator [2].Conclusion
We carried out first tests and high spatially-resolved MR measurements to determine the performance and feasibility of using a quadrature 19F CRP for application in future in vivo studies. An SNR gain exceeding 6 compared with a state-of-the-art room temperature 19F RF coil facilitated the acquisition of better spatially-resolved images. Preliminary results in ex-vivo mouse brain show the superiority of the quadrature CRP to delineate intricate areas of immune cell infiltration, thereby inspiring future in-vivo investigations in neuroinflammatory disease.Acknowledgements
This work was supported by a grant of the Deutsche Forschungsgemeinschaft (DFG) to Sonia Waiczies and Andreas Pohlmann.References
[1] Stadelmann C et al. (2011) Curr Opin Neurol 24: 224-229.
[2] Waiczies H et al. (2013) Sci Rep. 3: 1280.
[3] Waiczies H, Lepore S et al. (2011) PLoS ONE 6(7): e21981.
[4] Lepore S, Waiczies H et al. (2013) PLoS ONE 8(8): e72841.
[5] Baltes C et al. (2009) NMR Biomed 22(8), 834–842
Figures