4443
Ultrasound-based Cardiac Gating for MRI1Department of Radiology, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, United States
Synopsis
Clinical cardiac MRI typically requires the use of electrocardiogram (ECG) leads to detect R-waves and synchronize the MRI acquisition accordingly. The magnetohydrodynamic effect can corrupt the ECG signal, sometimes making R-wave detection difficult, especially at higher field strengths. We are proposing here a practical and low cost ultrasound-based solution for detecting cardiac activity in a manner immune to the presence and strength of the B0 field. Equivalence was shown in cases where ECG gating worked well. In the future, the approach should prove most helpful as an alternative to ECG when the latter proves impractical, especially at higher field strengths.
Introduction
Generating images that capture one or more specific cardiac phase(s) requires to monitor cardiac activity and synchronize the MRI acquisition accordingly. The standard for cardiac gating is electrocardiography (ECG), which senses the heart muscle’s electrophysiologic pattern using electrodes placed on the skin. However, depending on patient condition and scanner field strength, situations occur when ECG monitoring may not be a viable option. For example, in patients with prominent T waves, MRI acquisition may get erroneously triggered by T wave signals rather than by R waves. Alternately, the strength of the captured ECG signal may be impractically weak in some patients. Furthermore, ECG may get severely distorted due to the magnetohydrodynamic effect, especially at higher field strengths. Proposed alternatives to ECG include pulse gating, which is widely available, however bearing its own disadvantages mainly due to the time delay required for a pressure wave to make its way from the heart to the hand. Navigator echoes can be employed1, but they negatively affect scan time and may cause saturation artifacts in the image. A Cardiotocogram has been used for fetal cardiac MRI2. An acoustic solution has also been proposed, whereby sounds of the heart are used for triggering3, which is not trivial inside a noisy MRI system. The purpose of this work is to investigate the use of a custom 1 MHz single-element ultrasound transducer, referred to here as an organ-configuration motion (OCM) sensor, for echocardiography-based cardiac MR-triggering. The sensor was placed over the heart, and the resulting signal was processed to generate triggers at the end of atrial systole, at the onset of isovolumic contraction. The device is MR compatible and meant to replace ECG gating when the latter proves impractical, for the purpose of cardiac MRI.Methods
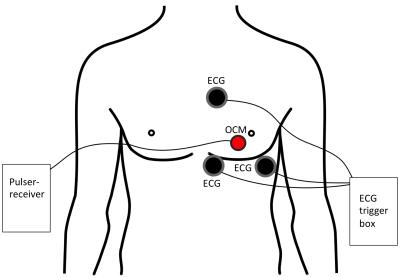
A pulser-receiver (5077PR, Olympus) triggered the 1MHz transducer at a pulse repetition frequency of 100Hz. The signal was recorded using a standard PC equipped with a digitizer card (5122, National Instruments), acquiring 5000 samples at 20MS/s, for a maximum depth of 19.25cm. The sensor was attached to the chest, directly over the heart, see Fig. 1. Three ECG leads were placed in the standard manner, see Fig. 1. Simultaneous OCM and ECG data were acquired, with the volunteer outside and then inside the 3T scanner, for 2 minutes each. The R-wave detection algorithm of the scanner, as applied to the minimally-distorted ECG traces acquired outside the bore, produced ground truth for validation purposes. A learning-based algorithm detected each heartbeat as follows. 200 OCM samples were selected around a depth of 16.5 cm. The mean absolute difference between subsequent OCM traces was used as a feature for heartbeat detection: $$ F(t) = \frac{\sum_{i=1}^{200} OCM_t(i) - OCM_{t-1}(i)}{200}. $$From the first 10 seconds, the standard deviation $$$\sigma$$$ of $$$F(t)$$$ was as learned, and during application, all instances above $$$1.2 \cdot \sigma$$$ were accepted as candidates for cardiac triggers. Because this method yields a number of spurious candidates, a stationary poisson point process with intensity $$$\lambda = 0.125$$$, corresponding 0.8s, was used to select a single trigger per cycle.Results
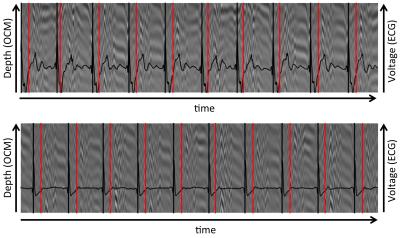
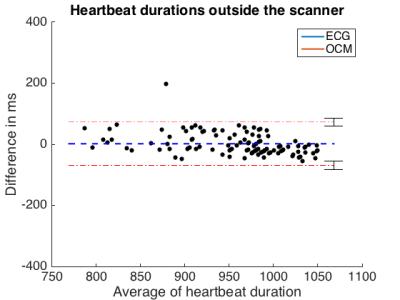
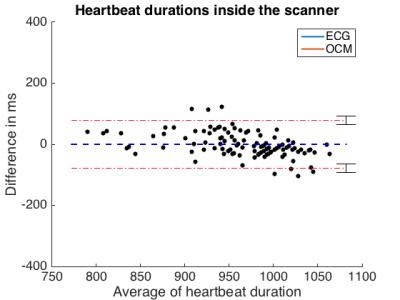
Figure 2 compares OCM and ECG from outside and inside the MR bore. Outside, 115 heartbeats were detected by both modalities. Mean heartbeat duration and standard deviation were 958 ms (± 577 ms) for ECG and 956 ms (± 716 ms) for OCM. Inside the scanner, these values were 964 ms (± 506 ms) for ECG and 963 ms (± 675 ms) for OCM (Figure X). Mean temporal deviation of each OCM trigger compared to its corresponding ECG trigger was -20 ms (± 3 ms) outside the scanner, -18 ms (± 3 ms), inside the scanner. Figures 3 and 4 compare the duration of each heartbeat as detected by ECG and OCM.
Results and Conclusion
We proposed a novel approach for cardiac triggering inside the MR bore that is not susceptible to the magnetohydrodynamic effect. The correct number of heartbeats was detected using our approach, as compared to ECG, and detected heartbeat duration proved very similar. A consistent shift between ECG and OCM triggers might indicate a systematic error, possibly due to a synchronization offset of the data streams, or more likely because the R-wave occurs slightly prior to the observable rapid motion of the heart detected by OCM. Such systematic shift could readily be compensated for if necessary. The present study was aimed at testing equivalence in cases where the ECG R-wave detection works well, future work should be directed at proving superiority in cases where ECG detection does not work well, which happens more frequently at higher field strengths.Acknowledgements
Support from NIH grants P41EB015898, R01EB010195 and R01CA149342 and SNSF grant P300P2_164647 is acknowledged.References
1. J N Oshinski, L Hofland, S Mukundan, Jr, W T Dixon, W J Parks, and R I Pettigrew, Two-dimensional coronary MR angiography without breath holding., Radiology 1996 201:3, 737-743
2. Wedegaertner, U., Frisch, M., Kopp, I., Graessner, J., Hecher, K., Adam, G., & Yamamura, J. A Novel Technique for Cardiac MRI of the Fetal Heart: MR compatible Doppler Ultrasound (CTG) for CardiacTriggering.
3. Frauenrath, T., Hezel, F., Renz, W., de Geyer d’Orth, T., Dieringer, M., von Knobelsdorff-Brenkenhoff, F., Prothmann, M., Schulz-Menger, J., Niendorf, T. (2010). Acoustic cardiac triggering: a practical solution for synchronization and gating of cardiovascular magnetic resonance at 7 Tesla. Journal of Cardiovascular Magnetic Resonance, 12(1), 1.
Figures