4429
Prescan consistency of ZTE-based attenuation map generation1GE Healthcare, Cambridge, United Kingdom, 2Department of Radiology, University of Cambridge, United Kingdom, 3GE Global Research, Germany, 4GE Healthcare, United States
Synopsis
We present an evaluation, across multiple clinical sites, of the impact of prescan variability on head attenuation maps based on zero echo time imaging.
Purpose
A new attenuation correction algorithm based on zero echo time (ZTE) data is currently being tested for clinical PET/MR imaging. By incorporating information about fast decaying species, this segmentation-based method can generate attenuation maps including bone density estimates, greatly improving the quantitative accuracy of the reconstructed PET data1,2,3.
Due to its impact in a quantitative modality such as PET, the consistency requirements for these ZTE-based attenuation maps are more stringent than for typical MRI sequences. The present study was designed to evaluate the impact of prescan variability on the acquired ZTE data and derived attenuation maps. Said variability was studied both in time and across systems, to assess the potential impact on longitudinal and multicenter studies, respectively.
Methods
Thirty ZTE acquisitions from four different sites equipped with a GE SIGNA PET/MR system were retrospectively analyzed. The same acquisition protocol, optimized for attenuation correction, was used in all cases: 3D radial ZTE sequence, FOV 26x26x28 cm, resolution 2.4x2.4x2.4 mm3, FA 0.8 deg, BW ±62.5 kHz, NEX 4. Two radiofrequency coils were supported: the 8-channel high-resolution brain coil and the head and neck unit. The post-processing of these datasets was fully automated, consisting of bias correction, intensity remapping and partial volume (i.e. skin and cavity) correction.
Dedicated scans of healthy volunteers were performed additionally, using the same protocol, in order to determine the variability of the prescan parameters under constant acquisition conditions. These consisted of several series of ZTE acquisitions, including several scenarios: Use of smart prescan rules; complete automated prescan; and manual prescan covering a range of parameter settings.
Results
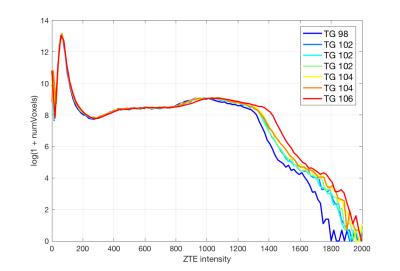
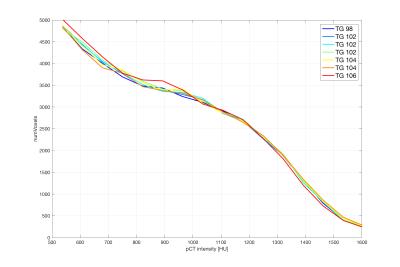
The prescan values of the ZTE sequence for the receive gain were found to be consistent across sites (13 for the analog gain and 29 for the digital gain). The transmit gain, on the other hand, showed significant differences between systems (e.g 118±9 on average with range [99, 130] vs 137±9 with range [123, 151]). In the case of pediatric patients, the transmit gain was 109±7 with range [100, 123] (being in this case loosely correlated with age: R2 = 0.53). Central frequency values were of course not comparable between sites.
For a given patient, the transmit gain was found to change noticeably between acquisitions when the full prescan procedure was repeated, even in the absence of repositioning (~2% standard deviation). Receiver gains stayed constant and central frequency showed negligible changes within the resolution of the measurement.
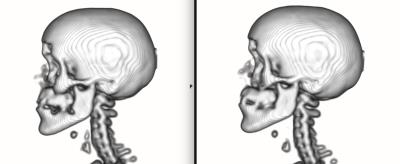
The bone maps obtained from repeated acquisitions of a patient displayed the same variability when the prescan procedure was repeated and when the prescan parameters were kept constant. In both cases, the differences between the bone maps (~1% on ray projections) were well below those caused by sub-voxel involuntary motion.
Discussion
A consistent behavior over the expected range of system states is mandatory for the clinical deployment of a prototype. The present study was prompted by a single reported instance of failed bone map generation, the cause of which was identified as an off-norm transmit gain value. To this day we have been unable to reproduce the issue. However, it shows the importance of characterizing the variability of prescan parameters for ZTE-based attenuation map generation.
While this may seem like a trivial question to an MRI audience –prescan variability being a known and indeed desired feature intended to optimize imaging conditions–, the impact of these subtle changes on bone density estimates deserves investigation due to the high accuracy demanded by quantitative PET applications. Indeed, prescan results are one of the few uncontrolled variables in ZTE-based attenuation correction, all other sequence parameters being fixed by design.
Despite the reported transmit gain variability and the evidence, provided by the retrospective analysis, that it might not be possible to use fixed thresholds as early indicators of potential attenuation map failures, the results of repeated scanning show an encouraging robustness to gain variability.
Conclusion
The data gathered during this study suggests that prescan variability, and in particular transmit gain variability, has a minimal impact on ZTE-based attenuation maps. Further investigation is required to determine the operational range of the segmentation and intensity mapping algorithm.Acknowledgements
No acknowledgement found.References
1. Wiesinger et al.: ‘Zero TE MR bone imaging in the head’, Magn Reson Med. 2016 Jan;75(1):107-14.
2. Delso et al.: ‘Clinical evaluation of zero-echo-time MR imaging for the segmentation of the skull’, J Nucl Med. 2015 Mar;56(3):417-22.
3. Sekine et al.: ‘Clinical evaluation of ZTE attenuation correction for brain FDG-PET/MR imaging-comparison with atlas attenuation correction’, J Nucl Med. 2016 Jun 23. In press.
Figures