4422
A Compact Planar Triple-Nuclear Coil for Small Animal 1H, 13C, and 31P Metabolic MR Imaging at 14.1 T1Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States
Synopsis
The development and test of a triple-nuclear surface coil for simultaneous 1H, 13C, and 31P small animal imaging at 14.1 Tesla.
Introduction
Multi-nuclear radio-frequency (RF) coils at ultrahigh field strengths are challenging to develop due to the high operating frequency, increased electromagnetic interaction among the coil elements, and increased electromagnetic interaction between the coils and the subject. In this work, a triple-nuclear surface coil for 1H, 13C, and 31P for small animal metabolic imaging at 14.1 T is built by nesting three surface coil geometries: a lumped-element L-C loop coil, a butterfly coil, and a microstrip transmission line (MTL) resonator.Methodology
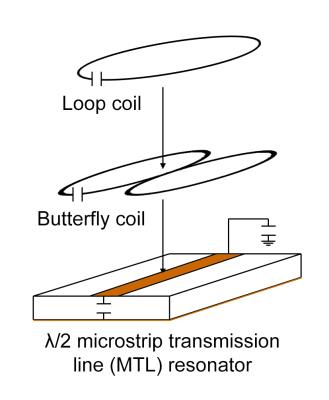
The triple-nuclear surface coil is built by nesting three surface coil geometries: a lumped-element LC loop coil, a butterfly1, and a half-wavelength (λ/2) microstrip transmission line (MTL)2. The three coil elements are arranged as shown in Figure 1.
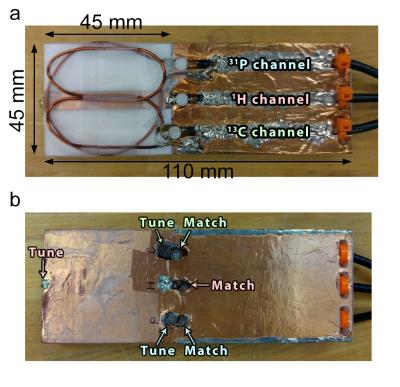
The MTL strip conductor (7mm×45mm×36μm) and the ground plate (50mm×45mm×36μm) is constructed with adhesive-backed copper tape on a PTFE substrate (45mm×45mm×7mm). Additional length (65mm) of the PTFE substrate was used to extend the ground plane and provide mechanical support for the coaxial cables. The MTL, loop coil, and butterfly coil, were tuned for 1H (600MHz), 31P (243MHz), and 13C (150MHz), respectively. The constructed coil is shown in Figure 2.
Bench-tests, tuning, and matching were done using a network analyzer (Agilent Technologies, Model E5061A). Scattering parameters, S11 and S21, and coil efficiency (Q-factor) were measured for both unloaded and loaded cases for each of the coil elements.
Two phantoms were imaged using a Varian 14.1T vertical bore micro-imaging scanner in separate scan sessions: a 2.5cm diameter sphere containing 5M enriched 13C-urea, and a 1.5cm diameter cylindrical sample tube containing 500mM sodium phosphate with a pH of 9.2.
The 13C-urea phantom was imaged using a 2D gradient-echo sequence (TE=1.92ms, TR=400ms, α=40°, Naverages=4, slice thickness=4mm, FOV=40 mm×40mm, matrix size=128×128) for the proton image, and a 2D chemical shift imaging (CSI) sequence (TE=0.53ms, TR=500ms, Naverages=2, spectral points=256, spectral width=4kHz, resolution=2.5mm×2.5mm, slice thickness=5mm, FOV=40mm×40mm) to obtain the 13C spectra.
The phosphate phantom was imaged using a 2D gradient-echo sequence (TE=1.88ms, TR=30ms, α=20°, Naverages=1, slice thickness=2mm, matrix size=128×128, FOV=50mm×50mm) to obtain the proton image, and a non-selective hard pulse (pulse width=180μs, α=90°, TR=6s, Naverages=16, complex points=10000, block size=8, spectral width=10kHz, acquisition time=984ms) to obtain the 31P spectrum.
The 13C and 31P spectral data were apodized using exponential filters to give line-broadening factors of 9 Hz and 20 Hz, respectively. The peak SNR (PSNR) is measured for each image and line spectra. The PSNR is measured by taking the maximum signal and dividing by the standard deviation of the background signals. For MRSI, the spectral SNR is measured for each voxel by taking the integral under the peak; the PSNR is taken from the voxel with the highest SNR. Triangle thresholding is used to differentiate real signal vs. background noise.
Results
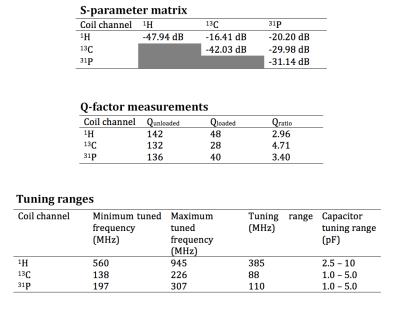
Bench test measurements are summarized in Figure 3. The coil achieves a worst-case S11 of -31.14 dB and S21 of -16.41 dB indicating good matching and decoupling performance.
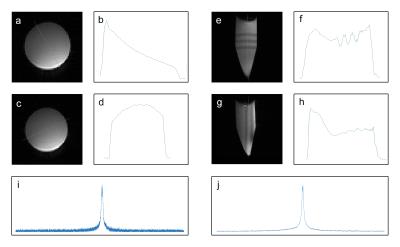
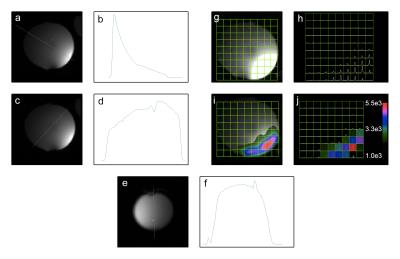
Results of the phosphorus phantom experiment are shown in Figure 4. The line profiles display extensive sample coverage, as expected from a MTL resonator. The peak SNR for the phosphorus proton image is 223. The spectral SNR is 8979 and 16062 for the raw and apodized spectra, respectively.
Results of the carbon-13 phantom experiment are shown in Figure 5. The peak SNR for the carbon-13 proton image is 452. The spectral peak SNR is 307 and 373 for the raw and apodized spectra, respectively
Discussion
The triple-nuclear coil allows for simultaneous experiments of 1H, 13C and 31P, which opens up avenues of research into the correlation of different metabolites with these nuclei such as for pH imaging3,4 or for cell energetics5,6. The coil may also be designed to work with other combinations of nuclei, such as 1H/23Na/39K, provided that the butterfly coil tuning has sufficient frequency separation with the MTL resonator tuning. Given the simple and compact structure, this triple-nuclear coil design can be a potential design block for multi-channel triple-nuclear RF coil array design if the appropriate broadband decoupling mechanism, such as ICE decoupling technique, is applied.Conclusion
A design for a triple-nuclear coil for 1H, 13C, and 31P small animal imaging at 14.1 T was successfully constructed and tested with the standard RF testing methods and MR imaging experiments. High Q-factors, extensive proton coil coverage, and sufficient decoupling for simultaneous 1H, 13C, and 31P imaging can be achieved with the proposed design.Acknowledgements
No acknowledgement found.References
1. Alfonsetti M, Sotgiu A, Alecci M. Design and testing of a 1.5 Tesla double-tuned (1H/31P) RF surface coil with intrinsic geometric isolation. Measurement. 2010;43(9):1266-1276. doi:10.1016/j.measurement.2010.07.003.
2. Pang Y, Xie Z, Xu D, et al. A dual-tuned quadrature volume coil with mixed λ/2 and λ/4 microstrip resonators for multinuclear MRSI at 7 T. Magn Reson Imaging. 2012;30(2):290-298. doi:10.1016/j.mri.2011.09.022.
3. Brindle KM, Rajagopalan B, Williams DS, et al. 31P NMR measurements of myocardial pH invivo. Biochem Biophys Res Commun. 1988;151(1):70-77. doi:10.1016/0006-291X(88)90560-8.
4. Lau AZ, Miller JJ, Tyler DJ. Mapping of intracellular pH in the in vivo rodent heart using hyperpolarized [1-13C]pyruvate. Magn Reson Med. April 2016:n/a-n/a. doi:10.1002/mrm.26260.
5. Brown R, Lakshmanan K, Madelin G, Parasoglou P. A nested phosphorus and proton coil array for brain magnetic resonance imaging and spectroscopy. NeuroImage. 2016;124, Part A:602-611. doi:10.1016/j.neuroimage.2015.08.066.
6. Janssen BH, Lassche S, Hopman MT, Wevers RA, Engelen BGM van, Heerschap A. Monitoring creatine and phosphocreatine by 13C MR spectroscopic imaging during and after 13C4 creatine loading: a feasibility study. Amino Acids. 2016;48(8):1857-1866. doi:10.1007/s00726-016-2294-0.
Figures