4408
Co-localization of individual neuroanatomical structures and intracranial electrodes to assist brain mapping for pre-surgical evaluation of epilepsy1National Chiao Tung University, Hsinchu, Taiwan, 2Chung Shan Medical University and Chung Shan Medical University Hospital, Taichung, Taiwan
Synopsis
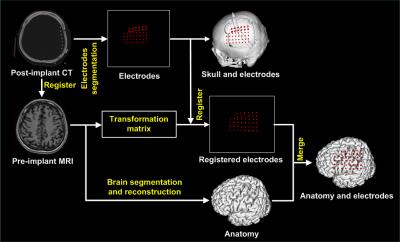
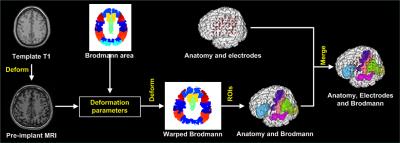
We proposed a method to precisely visualize subdural strip and grid electrodes in relation to underlying Brodmann’s area labeled cortical gyration. We transformed a reference brain labeled with Brodmann’s areas to match individual brain. Then we registered the digitalized electrodes from post-implant CT onto the anatomically labeled brain MRI.
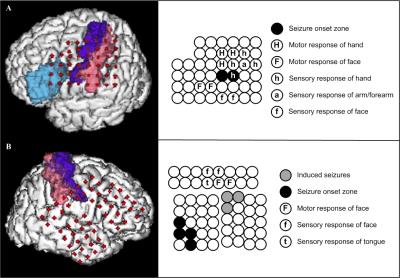
PURPOSE: A candidate for epilepsy surgery must haven’t attained acceptable seizure control with optimal trials of antiepileptic medication and have a reasonable change of benefiting from surgery. Not to mention new interventional methods, the outcome of conventional resective surgery relies on successful resection of epileptogenic zone in where responsible for the generation of focal seizures. Accurate localization of the epileptogenic zone is based on comprehensive pre-surgical evaluation that include neurophysiology and neuroimaging. Ideally, when the seizure focus can be precisely localized preoperatively, good surgical outcome will be obtained. The study is aimed to improve a technique investigation in epilepsy brain mapping for staged epilepsy surgery. METHODS: Two patients with drug-resistant epilepsy were included in our study from Chung Shan medical center. The seizure foci of the subjects were possibly located on the language or sensorimotor cortex by seizure semiology and ictal EEG. Therefore, all subjects had a procedure of subdural electrodes implantation for epilepsy surgery. Craniotomy and intracranial electrodes placement were planned relaying on the findings of multimodal neuroimaging and long-term scalp video EEG. CT scans for each patient were performed immediately after placement of electrodes before being transferred to the epilepsy monitoring unit for intracranial EEG monitoring. In addition, direct electrical stimulation of cortex was conducted to localize the function of specific brain regions. CT images were acquired with an LSVCTGE, 64 detectors unit using 32cm FOV; 512 × 512 Matrix and 0.625 mm slice thickness with isotropic reconstruction volume at 1mm. A high-resolution axial 3D T1-weighted structure image was acquired prior to electrode implantation using a fast-spoiled gradient-recalled echo (SPGR) sequence with the following parameters: TR/TE/TI = 2000/2.27/902 ms, Flip angle 8 degree, matrix 256 × 256, FOV 256 × 256 mm, and 160 slices. Visualizing implanted intracranial electrodes, epileptogenic zones and eloquent brain regions in three-dimensional space can greatly aid in planning, executing, and validating resection in surgical treatment of drug resistant epilepsy patients. We developed a methodological procedure intended to avoid the requirement for highly specialized medical resources and reduce time to identify the neuroanatomical location of epileptogenic zones. The co-localizing algorithm uses pre-implant MRI and post-implant CT fusion for the localization of epileptogenic zones and its relation with automatic labeled surrounding cortex. To validate our method; we utilize cortical electrical stimulation to assess electrodes overlying functional cortical fields corresponding to motor, somatosensory or language cortex. RESULTS: We successfully made 3D surface visualization of individualized colorfully labeled cortices overlaid with electrode for all subjects. Although the post-implant brains were partly deformed as a consequence of the craniotomy and subsequent electrode implantation, direct electrical stimulation of brain determined that personalized template parcellation of cortices conformed to known structural anatomy, serving as a good reference for localization of cortical functions. DISCUSSION: The co-labeling method will also combine neuroanatomical structures with functional activation further. Therefore, the technique is designed to image seizure source for epileptologists and neurosurgeons to conduct epilepsy surgery. CONCLUSION: We proposed a method to precisely visualize subdural strip and grid electrodes in relation to underlying Brodmann’s area labeled cortical gyration. We transformed a reference brain labeled with Brodmann’s areas to match individual brain. Then we registered the digitalized electrodes from post-implant CT onto the anatomically labeled brain MRI.
PURPOSE: A candidate for epilepsy surgery must haven’t attained acceptable seizure control with optimal trials of antiepileptic medication and have a reasonable change of benefiting from surgery. Not to mention new interventional methods, the outcome of conventional resective surgery relies on successful resection of epileptogenic zone in where responsible for the generation of focal seizures. Accurate localization of the epileptogenic zone is based on comprehensive pre-surgical evaluation that include neurophysiology and neuroimaging. Ideally, when the seizure focus can be precisely localized preoperatively, good surgical outcome will be obtained. The study is aimed to improve a technique investigation in epilepsy brain mapping for staged epilepsy surgery. METHODS: Two patients with drug-resistant epilepsy were included in our study from Chung Shan medical center. The seizure foci of the subjects were possibly located on the language or sensorimotor cortex by seizure semiology and ictal EEG. Therefore, all subjects had a procedure of subdural electrodes implantation for epilepsy surgery. Craniotomy and intracranial electrodes placement were planned relaying on the findings of multimodal neuroimaging and long-term scalp video EEG. CT scans for each patient were performed immediately after placement of electrodes before being transferred to the epilepsy monitoring unit for intracranial EEG monitoring. In addition, direct electrical stimulation of cortex was conducted to localize the function of specific brain regions. CT images were acquired with an LSVCTGE, 64 detectors unit using 32cm FOV; 512 × 512 Matrix and 0.625 mm slice thickness with isotropic reconstruction volume at 1mm. A high-resolution axial 3D T1-weighted structure image was acquired prior to electrode implantation using a fast-spoiled gradient-recalled echo (SPGR) sequence with the following parameters: TR/TE/TI = 2000/2.27/902 ms, Flip angle 8 degree, matrix 256 × 256, FOV 256 × 256 mm, and 160 slices. Visualizing implanted intracranial electrodes, epileptogenic zones and eloquent brain regions in three-dimensional space can greatly aid in planning, executing, and validating resection in surgical treatment of drug resistant epilepsy patients. We developed a methodological procedure intended to avoid the requirement for highly specialized medical resources and reduce time to identify the neuroanatomical location of epileptogenic zones. The co-localizing algorithm uses pre-implant MRI and post-implant CT fusion for the localization of epileptogenic zones and its relation with automatic labeled surrounding cortex. To validate our method; we utilize cortical electrical stimulation to assess electrodes overlying functional cortical fields corresponding to motor, somatosensory or language cortex. RESULTS: We successfully made 3D surface visualization of individualized colorfully labeled cortices overlaid with electrode for all subjects. Although the post-implant brains were partly deformed as a consequence of the craniotomy and subsequent electrode implantation, direct electrical stimulation of brain determined that personalized template parcellation of cortices conformed to known structural anatomy, serving as a good reference for localization of cortical functions. DISCUSSION: The co-labeling method will also combine neuroanatomical structures with functional activation further. Therefore, the technique is designed to image seizure source for epileptologists and neurosurgeons to conduct epilepsy surgery. CONCLUSION: We proposed a method to precisely visualize subdural strip and grid electrodes in relation to underlying Brodmann’s area labeled cortical gyration. We transformed a reference brain labeled with Brodmann’s areas to match individual brain. Then we registered the digitalized electrodes from post-implant CT onto the anatomically labeled brain MRI.Acknowledgements
This work was supported in part by the Ministry of Science and Technology, Taiwan, under the project MOST 105-2218-E-009 -027 and in part by the "Aim for the Top University Plan" of National Chiao Tung University and the Ministry of Education, Taiwan.References
1. Azarion AA, Wu J, Pearce A, et al. An open-source automated platform for three-dimensional visualization of subdural electrodes using CT-MRI coregistration. Epilepsia. 2014;55(12):2028-2037.
2. Princich JP, Wassermann D, Latini F, et al. Rapid and efficient localization of depth electrodes and cortical labeling using free and open source medical software in epilepsy surgery candidates. Front Neurosci. 2013;7:260.
3. Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95-113.
4. Klein A, Andersson J, Ardekani BA, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46(3):786-802.
Figures