4405
Unified Proton and Fluorine Imaging of Small and Low Spin Density Samples at a Human Whole-Body 7 T MRI1Department for Biometrics und Medical Informatics, Otto-von-Guericke University, Magdeburg, Germany, 2Department of Electronics and Information Engineering, and Korea Artificial Organ Center, Korea University, Seoul, Korea, Republic of, 3Bioimaging Research Team, Korea Basic Science Institute, Ochang, Korea, Republic of, 4Department of Biomicrosystem Technology and Korea Artificial Organ Center, Korea University, Seoul, Korea, Republic of
Synopsis
In order to provide a system, which allows imaging of 19F MR contrast agents, an in-house-built 19F/1H transmit/receive system for 7 T was successfully tested in a human whole-body 7 T MRI system. This system enables the measurement of concentrations of 1.85 mM. For this approach we used a 19F tuned coil which provided still enough signal gain at the proton frequency to allow 1H imaging for comparison. This showed the possibility of using 19F as contrast agents with a quite simple coil design in comparison to other dual tuned approaches.
Introduction
The number of fluorinated substrates used as pharmaceutical drugs has increased considerably during the last years1. For medical chemistry and therapy, fluorinated substrates are of high interest, because of the very low natural abundance of fluorine in living organisms leading to background-free 19F images. Furthermore, the high MR sensitivity predestines fluorine for 19F MRS and MRI examinations as a part of contrast agents, e.g. for metabolism studies of pharmaceuticals by using MR techniques2,3. However, to date, studies of 19F MRI at 7 T are rare due to the lack of existing coils4. Therefore, we have developed and successfully tested in-house-built 19F/1H transmit/receive system for 7 T imaging in a proof of principle study. In order to use such a system for detection of contrast agents it is important to be able to detect small volumes as well as small concentrations of the investigated substrates. To exploit the potential for fast imaging, for short TE-substrates containing low concentrations of 19F such as solid TEFLON cases, and for optimizing SNR ultra-short TE sequences were adapted to 19F imaging.Methods
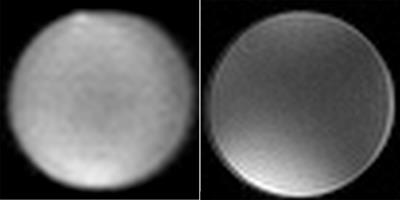
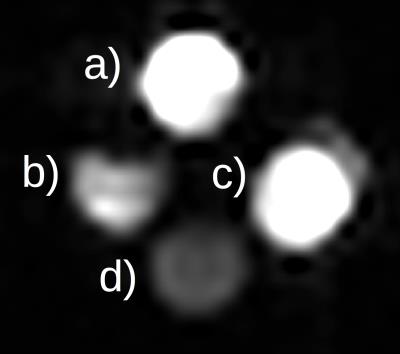
Measurements were performed with a human whole-body 7 T MRI system (Siemens, Erlangen, Germany). The transmit/receive coil and the casing were designed and build in-house (Fig. 1). The 4 array-elements of the coil were capacitively decoupled and matched to 279.5 MHz, which is the Larmor frequency of 19F at 7 T. The coil elements and the transmit-box were constructed to optimize the 19F-signal at a still sufficiently high gain of the 1H-signal for imaging and shimming. The volume coil had an inner diameter of 6.7 cm. The study used different phantoms: (i) a pure 2,2,2-trifluoroethanol (TFE, C2H3F3O) bottle phantom of 4 cm diameter and (ii) 15 ml falcon tubes with different concentrations of TFE dissolved in H2O (distilled water) between 926.9 mM and 1.85 mM (Fig. 2). For comparison between the obtained 1H to 19F imaging two different sequences were used: UTE (Ultrashort Time of Echo) and FLASH (Fast Low Angle Shot) sequences for imaging (for sequence parameters see figure captions). Both standard 1H sequences were adapted for 19F.Results and discussion
The sensitivity of the coil-transmit-box unit provided sufficiently high signal intensities both at 19F and 1H which enabled shimming at 1H and imaging at 1H and 19F frequencies even in case of 100% trifluoroethanol phantom. The phantoms showed good SNR and image homogeneity (Fig. 3 and 4) in the 1H and the 19F images. This demonstrates, that imaging for 19F and 1H substrates at a 7 T human scanner is feasible. Except for the volume of the imaged object the spin density and substrate concentration plays an important role when using 19F as a molecular marker substance or contrast agent. In our case we could image concentrations down to 1.85 mM, which is under the sensitivity required for contrast agents5 (Fig. 4). Thus, the coil architecture and the increased signal at 7 T show, that small amounts of 19F-labeled substances are well detectable potentially enabling the therapeutic monitoring of 19F drugs or using 19F-labeld substances as new contrast agents. The current system can be optimized in decoupling and contacts, which will lead to an even better sensitivity. This means that the diameter of the coil can be extended to a size of a head, which is important for further studies with 19F drugs.
So far, we have only used the 1H signal for shimming; an optimization of the 19F shimming is currently under investigation.
Using the same coil elements for simultaneous 19F and 1H measurements represents an advantage compared to complex dual tuned coils as no additional manipulation is necessary when switching between 1H and 19F. Besides this, the results show that the signal from 1H is still high enough to enable anatomic imaging (Fig. 3) allowing to use both image information, e.g., to depict 19F information on standard anatomy.
This study used trifluoroethanol as a 19F substrate due to its solubility in water. However, other 19F substances can be similarly used. Other non-toxic substances are now under investigation.
Conclusion
The results show that our concept of a phased-array coil with sufficiently broad frequency range allows for 1H and 19F imaging of fluorinated substances at a 7 T whole-body system designed for use of human in-vivo examination even if spin density are low. As 7 T will soon be available for clinical diagnostics this may provide new possibilities to monitor fluorinated drugs used in tumor therapy or treatment of psychiatric diseases.Acknowledgements
This work was supported by the BMBF Project 01DR12111: "EDUHF-LAB MRI“ - A German-Korean laboratory for training, development and research in ultra-high field whole-body MRI-technology.References
1. Wang J, Sánchez-Roselló M, Aceña JL et al. Fluorine in pharmaceutical industry: fluorine-containing drugs introduced to the market in the last decade (2001-2011). Chem. Rev. 2014; 114: 2432−250.
2. Ruiz-Cabello J, Barnett BP, Bottomley PA et al. Fluorine (19F) MRS and MRI in biomedicine. NMR Biomed. 2011; 24(2): 114-129.
3. Jiang Z-X, Liu X, Jeong E-K et al. Symmetry-guided Design and Fluorous Synthesis of A Stable and Rapidly Excreted Imaging Tracer for 19F MRI. Angew. Chem. Int. Ed. 2009; 48: 4755–4758.
4. Amiri H, Srinivas M, Veltien A et al. Cell tracking using 19F magnetic resonance imaging: Technical aspects and challenges towards clinical applications. Eur. Radiol. 2015; 25: 726–735.
5. Kenny GD, Shaw KP, Sivachelvam S et al. A bisphosphonate for 19F-magnetic resonance imaging. J. Fluor. Chem. 2016; 148: 58-64.
Figures