4400
Density Weighted Concentric Rings K-Space Trajectory for 1H MRSI with gradient offset independent adiabatic pulses at 7T1Oxford Centre for Functional MRI of the Brain (FMRIB), University of Oxford, Oxford, United Kingdom, 2UC Berkeley-UCSF Graduate Program in Bioengineering, University of California, Berkeley and University of California, San Francisco, CA, United States, 3Department of Radiology and Biomedical Imaging, University of California, San Francisco, CA, United States, 4Department of Oncology, University of Oxford, Oxford, United Kingdom, 5Department of Radiological Sciences, University of California, Los Angeles, CA, United States
Synopsis
In this study, we have developed and demonstrated a GOIA-semi-LASER sequence with density-weighted (DW)-concentric rings trajectory (CRT) that performs robustly at 7 Tesla and within a clinically feasible acquisition time. DW-CRT has been validated in a series of phantom experiments and its feasibility assessed in a healthy volunteer with an in-plane resolution of 5×5 mm2. Experiments qualitatively demonstrate the advantage of DW-CRT over uniformly-weighted (UW)-CRT in terms of its improved resolution and reduced contamination of spectra from neighboring voxels.
Introduction
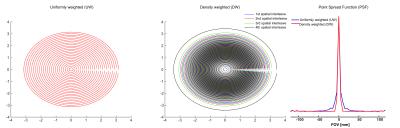
Magnetic resonance spectroscopic imaging (MRSI) offers the advantage of non-invasively collecting data of a tissue’s neurochemical profile over larger regions than single-voxel MRS and at high spatial resolutions. However, the practicality of conventional phase-encoded MRSI methods is compromised by the long acquisition times required for spatial encoding. At ultrahigh fields (UHFs), such as 7 T, MRSI benefits from increased signal-to-noise ratio (SNR), as well as the possibility to quantify more metabolites as a result of a better separation of neighboring resonances, and these gains could be translated into either higher spatial resolution or faster scanning (1). Together with UHF, fast phase encoding trajectories, both echo-planar (Echo-Planar Spectroscopic Imaging or EPSI (1)) and non-echo-planar (spiral (2), rosette (3) and concentric (4) spectroscopic imaging), have been utilized to reduce the acquisition time. The use of a concentric rings trajectory (CRT) is more time-efficient compared to EPSI, and less sensitive to system imperfections compared to spiral MRSI (5,6). Thus, in this study, we propose using a concentric rings trajectory in combination with gradient offset independent adiabatic (GOIA) semi-LASER as a 2D MRSI data acquisition method at 7T. In order to improve the point spread function (PSF) of the 2D-MRSI acquisition without any loss of SNR efficiency, a density-weighted (DW)-CRT sampling approach is adopted and compared with conventional density compensated uniformly weighted (UW)-CRT (7).Methods
All phantom and healthy volunteer measurements were conducted at 7T using a whole body MR system (Siemens, Erlangen) with a Nova Medical 32-channel receive array head-coil. B0 shimming was achieved using GRESHIM (8). The semi-LASER (9) localization using GOIA pulses (HS8.R50) (TR = 1.5 s, TE = 36 ms, was used to excite a 120 mm x 120 mm x 20 mm region centrally within the field of view (FOV= 240 mm x 240 mm). For UW and DW-CRT, 64 points per ring were collected with an ADC bandwidth of 80 kHz, resulting in 512 temporal samples in an effective spectral bandwidth (SBW) of 1250 Hz (Figure 1). Temporal interleaves were implemented by inverting the readout trajectory to increase SBW (2500Hz). To cover the 48x48 grid, 24 rings resulting in an individual voxel size of 0.5 mL were acquired for UW and DW-CRT. The weighting function, w(k) (Eq. 1) was used to compute radial sampling locations for the DW-CRT:
$$w(k)=∆x/2 (1+cos (2πk∆x/1.71))$$ Eq. 1
where Δx is the nominal spatial resolution. For DW-CRT, four spatial interleaves (96 unique rings) were used to ensure that minimum field of view requirements were met, resulting in an acquisition duration of 288s with temporal interleaves. The number of averages (NA) for the phantom acquisitions was 4 for UW-CRT, corresponding to the same acquisition duration with temporal interleaves. All reconstructions were implemented in MATLAB using the non-uniform FFT (NUFFT) toolbox (10).
Results
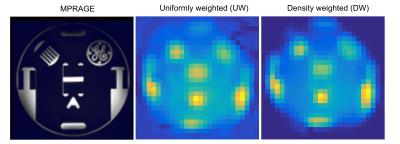
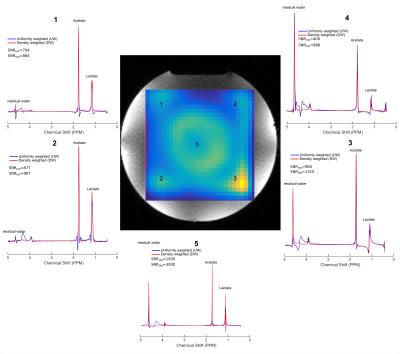
Simulations were performed to investigate the effect of the DW-CRT on the PSF. When compared to UW, DW-CRT resulted in aan improved PSF with strong sidelobe suppression (Figure 1). Improvement in PSF was later demonstrated experimentally on a cylindrical resolution phantom (General Electric Medical Systems) using the non-water suppressed MRSI acquisition, confirming that DW-CRT provided apparently enhanced spatial resolution compared to UW-CRT (Figure 2). Another phantom experiment to assess metabolite spectral quality was performed on a MRS phantom (Siemens, Erlangen). When compared to the UW-CRT, the improved the shape of the DW-CRT PSF resulted in substantially reduced spectral contamination in neighboring voxels, without any loss of SNR efficiency (Figure 3). In agreement with simulations and phantom measurements, in vivo measurement resulted in enhanced localization and increased SNR from avoiding post-hoc density compensation (Figure 4).Conclusion
We have developed and demonstrated a GOIA-semi-LASER sequence with DW-CRT that performs robustly at 7 Tesla and within a clinically feasible acquisition time. DW-CRT has been validated in a series of phantom experiments and its feasibility assessed in a healthy volunteer with an in-plane resolution of 5×5 mm2. Experiments qualitatively demonstrate the advantage of DW-CRT over UW-CRT in terms of its improved resolution and reduced contamination of spectra from neighboring voxels. Future work will focus on implementing the method in whole brain with a reduced TR.Acknowledgements
The authors would like to acknowledge the following: the Welcome Trust and the Dunhill Medical Trust.References
1. Otazo R, Mueller B, Ugurbil K, Wald L, Posse S. Signal-to-noise ratio and spectral linewidth improvements between 1.5 and 7 Tesla in proton echo-planar spectroscopic imaging. Magn Reson Med 2006;56(6):1200-1210.
2. Philips B, van de Stadt-Lagemaat MW, van Uden MJ, Vos EK, Gagoski B, Kerr AB, Maas MC, Scheenen TWJ. Spectral-Spatial-Spiral MRSI: Fast prostate MR spectroscopic imaging with low SAR on 7T. 2015; Toronto. p 4708.
3. Schirda C, Zhao T, Hetherington H, Yushmanov V, Pan J. Rosette Spectroscopic Imaging (RSI) of human brain at 7T. 2016; Singapore. p 2351.
4. Wilson N, Hariharan H, Thomas MA, Ravinder R. Concentrically circular echo planar spectroscopic imaging at 3T and 7T with partial temporal interleaving. 2016; Singapore. p 2352.
5. Jiang W, Lustig M, Larson PE. Concentric rings K-space trajectory for hyperpolarized (13)C MR spectroscopic imaging. Magn Reson Med 2016;75(1):19-31.
6. Furuyama JK, Wilson NE, Thomas MA. Spectroscopic imaging using concentrically circular echo-planar trajectories in vivo. Magn Reson Med 2012;67(6):1515-1522.
7. Greiser A, von Kienlin M. Efficient k-space sampling by density-weighted phase-encoding. Magn Reson Med 2003;50(6):1266-1275.
8. Shah S, Kellman P, Greiser A, Weale P, Zuehlsdorff S, Jerecic R. Rapid Fieldmap Estimation for Cardiac Shimming. Proceedings 17th Scientific Meeting, International Society for Magnetic Resonance in Medicine. Honolulu 2009. p 565.
9. van de Bank BL, Emir UE, Boer VO, van Asten JJ, Maas MC, Wijnen JP, Kan HE, Oz G, Klomp DW, Scheenen TW. Multi-center reproducibility of neurochemical profiles in the human brain at 7 T. NMR Biomed 2015;28(3):306-316.
10. Fessler JA, Sutton BP. Nonuniform fast Fourier transforms using min-max interpolation. IEEE Transactions on Signal Processing 2003;51(2):560-574.
Figures