4399
From medulla to lower cervical levels, a multi-parametric quantitative MR investigation dedicated to the diffuse alterations of the spinal cord at 7T: first insights into Amyotrophic Lateral Sclerosis1Aix-Marseille Université, CNRS, CRMBM UMR 7339, Marseille, France, Marseille, France, 2AP-HM, Hôpital de la Timone, Pôle d’imagerie médicale, CEMEREM, Marseille, France, Marseille, France, 3iLab-Spine - Laboratoire international associé - Imagerie et Biomécanique du rachis, France/Canada, Marseille, France, 4Siemens Healthcare GmbH, Erlangen, Germany, 5Service de Neurologie et Maladies neuromusculaires, Hôpital de La Timone, AP-HM, Marseille, France
Synopsis
Amyotrophic lateral sclerosis (ALS) may benefit from unique contrasts and high-spatial resolutions provided by ultra-high field brain MRI. This also stands for spinal cord; nevertheless spinal cord imaging at 7T has lagged considerably behind brain investigations until recently. In this work, we propose a multi-parametric quantitative MR imaging protocol (T1, T2*, DTI, CSA), in an acquisition time compatible with clinical research (50min), to comprehensively investigate spinal cord diffuse alterations in neurodegenerative diseases such as ALS. With imaging along the whole cervical cord and medulla, theses multi-parametric acquisitions have the potential to provide new insights for the study of ALS.
Introduction
Amyotrophic lateral sclerosis (ALS) is a devastating neurodegenerative disease characterized by progressive and rapid alteration of upper and lower motor neurons. Its diagnosis is predominantly clinical and based on electrophysiological criteria. With the ability of ultra-high field (UHF) MRI to provide exquisite images of the brain with unique contrasts at high spatial resolutions [1], ALS characterization could be improved [2]. Demonstrated in brain studies, tissue degradation related to ALS has also been shown in the spinal cord (SC) using multi-parametric MRI at lower field strength [3-4]. However, similar developments at 7T have lagged considerably behind so far, with only a few studies endeavored in volunteers and patients until now [5-9]. Most of these SC UHF studies relied on axial plane acquisition to avoid WM/GM partial volume effect, and focused on a specific cervical level (usually C3) for quantitative MR imaging (qMRI). Yet, to comprehensively investigate the SC diffuse alterations encountered in neurodegenerative diseases such as ALS, qMRI at each cervical level is needed. In this preliminary work based on five healthy volunteers and two ALS patients with bulbar-onset, we applied a high-resolution multi-parametric qMRI protocol covering the entire spinal cord and the medulla oblongata (MO) in an acquisition time (50min) compatible with clinical research. Automatic SC segmentation and registration to template enabled quantification of T1 and T2* relaxation times, DTI metrics and cross sectional areas (CSAs) within regional SC WM tracts and GM horns. Quantitative results were also obtained at the level of the MO, where small substructures could be well depicted.Methods
Setup: whole-body 7T system (Siemens, Erlangen, Germany) with an eight-channel transceiver cervical SC coil array.
Exams: 2 bulbar-onset ALS patients with little SC alterations (62/49y.o, 2F, ALSFRS scores: 43/35, disease durations: 1.2/3y) and 5 healthy volunteers (42±13y.o, 3M/2F) scanned with approval of the local Ethic Committee.
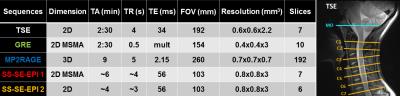
MR imaging with MO/C1-C7 coverage (see Figure1): T2-w TSE, T1-w MP2RAGE [10], T2*-w multi-echo GRE and diffusion-weighted single-shot SE-EPI using a prototype sequence (b-values: 0-800s/mm2, 20 dir, 3 averages, pulse triggered).
Total acquisition time, including manual system adjustments (B0 shimming, B1+ mapping): 50 min.
Post-processing: semi-automated, using Matlab (The Mathworks, Natick, MA, USA) and FSL (FMRIB, Oxford, UK) for image processing; PropSeg [11] for spinal cord segmentation and ANTs SyN [12] for non-linear registration of the images to the T2*-w AMU40 SC template [13].
Quantification: template-based [7;14] within specific SC WM and GM regions of interest (ROIs) at all cervical levels, manual delineation in MO.
Results
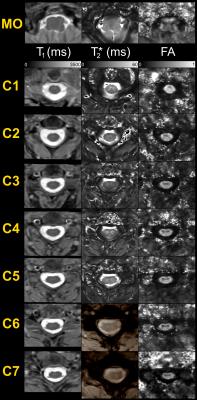
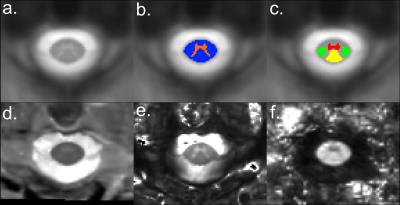
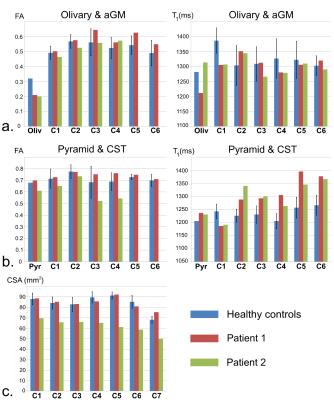
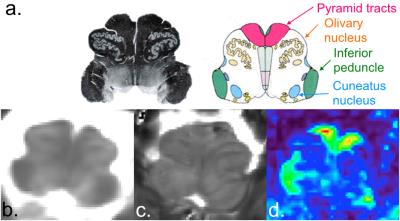
Figure2 exhibits a global panel of multi-parametric qMRI dedicated to the investigation of diffuse cord tissue alterations (T1 maps, T2* maps and FA maps) of MO (top row) and cervical levels acquired on a 57y.o. female volunteer (closest age-match to patients). Figure3 briefly illustrates multi-parametric data registration to the AMU40 template, enabling automatic quantification within defined ROIs, shown in color. Figure 4 summarizes some quantitative results (T1 and FA) obtained in anterior GM horns, in lateral motor WM tracts and in the MO. Some differences between healthy controls and patients could be seen, however no robust interpretations relevant to microstructural changes could be performed at this step given the small sample size. Finally, thanks to coil large coverage, MO substructures could also be clearly visualized. Figure 5 illustrates qMRI of the MO focusing on a bulbar-onset ALS patient, in line with UHF studies focusing on the brainstem [15].Discussion
A multi-parametric qMRI protocol is employed to set up ALS characterization in the SC at 7T.
High-resolution qMRI data are reported at all cervical levels and clear
delineations of the SC substructures enable quantification into specific ROIs
(anterior GM containing motor neuron cells; lateral corticospinal
tracts) that may be impaired in SC diseases. The proposed
acquisition time, which allows gathering complementary quantitative data, is
compatible with clinical research and is well tolerated by patients. Inclusion
of spinal-onset ALS patients is underway and will release the full potential of
such protocol. Due to large B1 inhomogeneities, C6 and C7 levels imaging is
not always satisfying. Refined coil design and parallel transmission imaging [16]
will contribute to break these locks. Imaging in lower cervical levels is also
seriously impaired by respiratory movements, which need to be dealt with. MT-based
acquisitions and MR spectroscopy, although technically challenging, are also highly
relevant to the study of structuro-functional impairments in ALS, and will hence
be considered as well in the future. Further work also includes increasing in-plane
resolution of both acquisitions and SC MR templates.
Altogether, this study shows that 7T is ready for clinical studies in
the SC involving serious neurodegenerative pathologies.
Acknowledgements
Fundings: ANR-11-EQPX-0001, A*MIDEX-EI-13-07-130115-08.38, A*MIDEX ANR-11-IDEX-0001-02.References
[1] Van der Zwaag et al., NMR Biomed 29, 2016. [2] Kwan et al., PLoS One 7, 2012. [3] El Mendili et al., PLoS One 9, 2014. [4] Rasoanandrianina et al., ISMRM 2016, p 1500. [5] Cohen-Adad et al., Muscle & Nerve, 2012. [6] Sigmund et al., NMR Biomed 25, 2012. [7] Massire et al., NeuroImage 143, 2016. [8] Dula et al., Mult Scler 2015. [9] Dula et al., NMR Biomed 2016. [10] Marques et al., NeuroImage 49, 2010. [11] De Leener et al., NeuroImage 98, 2014. [12] Avants et al., Med Image Anal 12, 2008. [13] Taso et al., NeuroImage 117, 2015. [14] Lévy et al., NeuroImage 119, 2015. [15] Deistung et al., Front. Human Neuro 7, 2013. [16] Zhao et al., MRM 72, 2014.Figures