4396
Textural Analysis based Segmentation of Bone tumours using Diffusion Weighted MR Image1Centre for Biomedical Engineering, Indian Institute of TechnologyDelhi, New Delhi, India, 2Department of Biomedical Engineering, All India Institute of Medicine, New Delhi, India, 3Centre for Biomedical Engineering, Indian Institute of TechnologyDelhi, 4Department of Radio Diagnosis, All India Institute of Medical Sciences, 5Institute-Rotary Cancer Hospital, All India Institute of Medical Sciences
Synopsis
Diffusion weighted imaging (DWI) plays a crucial role in diagnosis and prognosis of cancerous diseases. Proper demarcation of tumour on DWI is therefore necessary for qualitative assessment as well as quantitative image analysis like ADC or IVIM. Manual demarcation of tumour on each DWI slice is time consuming, prone to error and hard to reproduce. Automated and semi-automated algorithms were implemented and tested to segment bone tumours specifically on DWI. Experimental results reveal that semi-automated Active-Contours performed tumor segmentation better than other methods with acceptable levels of accuracy with considerable less time and manual effort.
Purpose:
Diffusion weighted imaging (DWI) plays a crucial role in diagnosis and prognosis of tumorus as it is capable to capture the cellular changes in the diseased tissue early in the course of treatment. Proper demarcation of tumour on DWI MRI is necessary for qualitative as well as quantitative image analysis like ADC or IVIM. Manual demarcation of tumour on each DWI slice is time consuming, prone to error and hard to reproduce. Automated tumour segmentation also becomes an essential part of the quantitative image analysis methodology in response evaluation of tumour to neoadjuvant chemotherapy. The purpose of this study was to evaluate automated and semi-automated algorithms that might be effective in segmenting bone tumors in DWI, with reasonable accuracy, speed and minimal manual input.Methods:
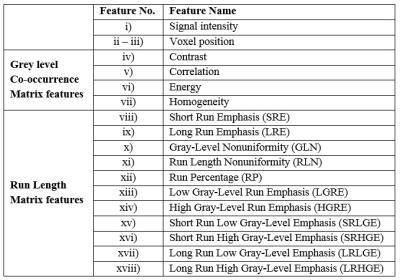
DWI dataset from six patients (Age=25.6±8.8yrs) with Ewing’s Sarcoma family of tumours (ESFT) and six patients with Osteosarcoma (Age=15.5±4.3yrs) were acquired under the Institutional protocol. The acquisition was performed using 1.5T Phillips Achieva MRI scanner with Spin Echo Planar imaging (SP-EPI) sequence with TE=66msec, TR=1782msec and 5mm slice thickness. Images with b=800s/mm2 were used for segmentation analysis. Following segmentation algorithms were implemented and examined in this study: a) Otsu-thresholding1, b) Neural Networks2 (NN), c) Otsu-thresholding based region growing3 (RG) and d) Active contours (Chan Vese method)4 (AC). Algorithms a)&b) were fully automated approaches that work without any additional human inputs and were intended to save time, or manual effort, or both. For NN, each image slice of DWI 3D stacks were pre-processed to primarily highlight the possible tumour area. Next, feature vector (length=18) comprising i) signal intensity, ii-iii) voxel position and textural features using iv-vii) Grey-level Co-occurrence Matrix5 and viii-xviii) Run Length Matrix6, were extracted and feed to NN for the final segmentation (Figure1). Feed-forward neural net with twelve nodes, hyperbolic tangent sigmoid transfer function and leave p-out cross-validation (p=1,2) was used in this study. Algorithms c)&d) were semi-automated approach intended to optimize between time, accuracy and manual effort. These algorithms require user input at the initiation of the process that was seed points (for RG) or a tumor Region of Interest (ROI) (for AC). The algorithms then initialize prospective seed points or tumour ROIs automatically in subsequent slices by calculating centroid from previously applied ROIs and continue tumour segmentation covering entire 3D anatomy. If the difference in segmented tumour size of two adjacent slices is large (>50%), it indicates error and user was requested again to confirm or correct the ROI and proceed for further segmentation.
Tumor ROIs demarked manually by a radiologist (clinical experience of >10 years in cancer imaging) were considered as standard tumour mask and used to calculate the error in the different segmentation algorithms applied. The accuracy measure used to compare the performance of these algorithms as Jaccard Index (JI) and Dice Coefficient (DC). Where, $$$JI=|A∩B|/|A∪B| ×100%, $$$ , and $$$DC=2 |A∩B|/(|A|+|B| )×100%$$$, where A and B are the areas demarcated as tumour by expert radiologist and segmentation algorithm respectively. Both JI and DC varies between 0-100, 0 indicates poor performance with 100% error and 100 indicates an ideal scenario 100% accuracy in segmentation. The segmentation algorithms and analysis were implemented in an in-house built toolbox in MATLAB.
Results:
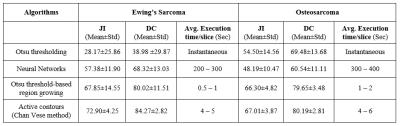
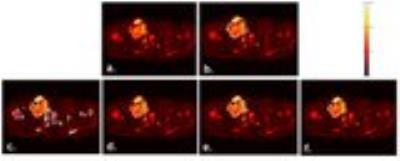
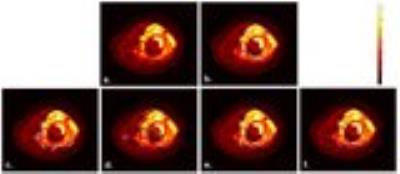
Figure2 shows the mean JI and DC for segmentation algorithms across DWI 3D stacks of all patients and the execution time per slice. Automated Otsu-thresholding produced output almost instantaneously, but with lower accuracy for ESFT (JI:~28%&DC:~39%) than Osteosarcoma (JI:~55% & DC:~70%). Automated NN produced better segmentation accuracy for ESFT (JI:~57%&DC:~68%) than Osteosarcoma (JI:~48%&DC:~61%) and took ~200-400sec execution time/slice. Semi-automated RG showed a moderately accurate result for both ESFT and Osteosarcoma, with average accuracy of JI:~66-68% and DC:~80% at execution rate of ~0.5-2 sec/slice. Semi-automated AC algorithm delivered marginally more accurate result than the other methods with accuracy of JI:~67-73% and DC:~80-84% for both diseases. It typically took ~4-6 seconds per slice. Illustrative examples of segmented images by four applied methods corresponding to the original images (Figure3a&4a) each from one representative patient with ESFT and Osteosarcoma are shown in Figure3&4 respectively. Automated NN, semi-automated RG and AC methods produced qualitatively more accurate segmentation than Otsu-thresholding.Discussion:
Among automated methodologies, NN produced better segmentation than Otsu-thresholding for ESFT although found less accurate for Osteosarcoma. Overall, semi-automated algorithms had better performance than automated methods with reasonable accuracy (JI: ̴ 66-73%&DC: ̴ 80-84%) and execution time/slice (<6sec) requiring manual intervention only at very few slices. Thus semi-automated segmentation methods may be more useful for bone tumors segmentation on DWI with acceptable level of accuracy.Acknowledgements
No acknowledgement found.References
1. Nobuyuki Otsu, IEEE Trans. Sys., Man., Cyber. 9 (1): 62–66, 1979.
2. Hornik Kurt, Neural Networks, Vol. 4, pp. 251-257, 1991.
3. T.F. Chan et al. IEEE-IP, 10 (2) : 266 – 277, 2001.
4. M. Mancas et al., (SPIE/EI 2005), San Jose (California, USA).
5. Haralick, R.M. et al. IEEE Trans. Syst., Man, Cybern., vol. SMC-3, pp. 610–621, 1973.
6. Galloway, M.M. Comput. Graphics Image Process., vol. 4, pp. 172–179, June 1975.
Figures