4393
Longitudinal diffusion MRI for treatment assessment of sarcoma patients with pre-operative radiation therapy1Radiation Oncology, UCLA, Los Angeles, CA, United States, 2Radiology, UCLA, Los Angeles, CA, United States
Synopsis
Diffusion weighted MRI (DWI) is promising for early prediction of tumor response to radiation therapy. We report our results of using longitudinal DWI approach performed on ViewRay system for predicting the response of sarcoma patient to pre-op RT. Six sarcoma patients were recruited in this study. Each patient subsequently underwent surgery. The tumor necrosis score was then used to compare to the ADC values to assess the predictive value of longitudinal DWI. Each patient successfully underwent 3–5 diffusion MRI scans. Based on the data from 6 patients, our longitudinal changes in tumor ADC matched well with pathology necrosis results.
Innovation/Impact:
Diffusion weighted MRI (DWI) is a promising imaging technique for early prediction of tumor response to radiation therapy 1,2. Recently, a longitudinal DWI approach has been proposed for tumor response assessment whereby the patient undergoes DWI on a MRI-guided radiotherapy (RT) system (MRIdian System™, ViewRay™, Cleveland, OH, USA) every 2-5 fractions 3. This strategy addresses several of the current scientific and practical challenges of using DWI for monitoring patient response to therapy and may bring functional MRI-guided adaptive RT closer to clinical utility. In this work, we present our preliminary results of using this longitudinal DWI strategy in conjunction with the ViewRay system for predicting response of sarcoma patients to pre-op RT.Introduction:
· Baseline apparent diffusion coefficient (ADC) or changes in ADC values between baseline and post-therapy time points have been shown to correlate with tumor control and patient outcome after radiotherapy 4-7.
· Currently, the diffusion MRI studies are often performed at baseline before initiation of treatment, and at a couple of time points during or sometimes several months after all the fractions are finished. The current time scale may not be sufficient to fully characterize the longitudinal tumor response curve over the treatment course and the time points at which adaptive therapy can potentially be used is also limited.
· Diffusion MRI data on a finer time scale to longitudinally track tumor response would be valuable for us.
· We have elected to acquire diffusion image data at every 2–5 treatment days throughout the multi-fraction RT. In this work, we report our early experience of diffusion MRI at the ViewRay 0.35T low field MRI system in a small cohort of sarcoma patients undergoing preop RT. We correlated our diffusion findings with pathology necrosis scores after their surgery to show DWI’s potential value of longitudinal tumor response assessment.
Methods:
Under an Institutional Review Board approved protocol, a total of six sarcoma patients were recruited in this study. All six patients underwent fractionated IMRT with prescription dose as following: Rx (patient 1) = 4Gy x 10fractions; Rx (patient 2) = 3.5Gy x 8fractions; Rx (patient 3, 4, 5) = 6Gy x 5fractions; Rx (patient 6) = 1.8Gy x 28fractions. For all six patients DWI images were acquired throughout their entire treatment course. For each imaging session, ten slices were acquired interleaved with different b-values covering the gross tumor volume (GTV), which was typically positioned near the isocenter. The diffusion images were processed to obtain the ADC maps for each slice using standard exponential fitting for each voxel. Regions of interest (ROIs) were drawn in the tumor on the diffusion images based on each patient’s clinical GTV contours. Average center slice ADCs within ROIs were plotted across fraction numbers when images were acquired.Results:
Each patient successfully underwent 3–5 diffusion MRI scans depending on their treatment length.
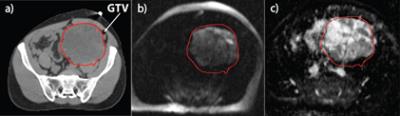
· Figure 1 shows Patient 1 with a 32 × 22 × 14 cm3 tumor. The simulation CT image [Fig. 1(a)] did not differentiate well between tumor and surrounding normal tissue. The diffusion-weighted image [Fig. 1(b), b = 500] clearly shows the enhancing tumor that matched well with the patient’s GTV contour. In the corresponding ADC map [Fig. 1(c)], there was considerable heterogeneity within the tumor. Patient 1 had a relatively unchanged ADC during the course of RT and a necrosis score of 30% at surgery.
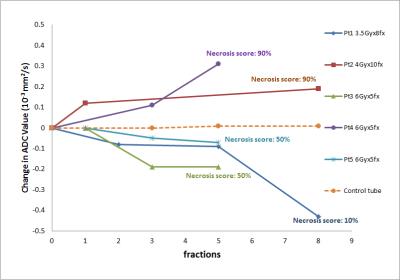
· For the remaining 5 patients, shorter RT treatment courses were used for patient convenience and also for less delay to the surgery. Longitudinal ADC changes for all five patients are shown in figure2. ADC values for patient1 dropped from 1.56x10-3 to 1.12x10-3 mm2/s during the course of treatment. Patient underwent biopsy 47 days after radiation therapy with a necrosis score of less than 10%, an indication of poor response to the treatment or tumor progression. Pathology results of patient2 and patient4 give a high necrosis score of 90%, an indication of good response to treatments. The curves of the average ADC change also show an increase throughout the treatment course for the same two patients. The longitudinal ADC curve of patient3 and patient5 have similar pattern, the initial decreasing curve followed by a flat section. This may indicate initial tumor progression then stable disease. The post-surgery pathology reports both show a necrosis score of 50%.
Conclusion:
Longitudinal diffusion MRI at the 0.35T ViewRay system is feasible. Based on our data from six patients, a decreasing (increasing) ADC value during the course of treatment corresponded to lower (higher) necrosis score from pathology.Acknowledgements
No acknowledgement found.References
[1] P. Bhatnagar et al.,“Functional imaging for radiation treatment planning, response assessment,and adaptive therapy in head and neck cancer,” RadioGraphics 33,1909–1929 (2013).
[2] C. Tsien et al., “Clinical applications for diffusionmagnetic resonance imaging in radiotherapy,” Semin. Radiat. Oncol. 24,218–226 (2014).
[3] Y. Yang et al., “Longitudinal diffusion MRI for treatment response assessment: Preliminary experience using an MRI-guided tri-cobalt 60 radiotherapy system,” Med. Phys. 43(3), 1369–1373 (2016).
[4] G. Decker et al., “Intensity-modulated radiotherapy of the prostate: DynamicADC monitoring by DWI at 3.0 T,” Radiother. Oncol. 113, 115–120(2014).
[5] J. Il Yu et al., “The role of diffusion-weighted magneticresonance imaging in the treatment response evaluation of hepatocellularcarcinoma patients treated with radiation therapy,” Int. J. Radiat. Oncol.,Biol., Phys. 89, 814–821 (2014).
[6] F. Kuang et al., “The value of diffusion-weightedMRI to evaluate the response to radiochemotherapy for cervical cancer,”Magn. Reson. Imaging 32, 342–349 (2014).
[7] S. Kim et al., “Diffusion-weighted magnetic resonance imaging for predictingand detecting early response to chemoradiation therapy of squamous cellcarcinomas of the head and neck,” Clin. Cancer Res. 15, 986–994 (2009).
Figures