4391
Distortion-Free Diffusion MRI using an MRI-Guided Tri-Cobalt 60 Radiotherapy System: Sequence Validation and Preliminary Clinical Experience1Radiology, University of California, Los Angeles, Los Angeles, CA, United States, 2Physics and Biology in Medicine IDP, University of California, Los Angeles, Los Angeles, CA, United States, 3Radiation Oncology, University of California, Los Angeles, Los Angeles, CA, United States
Synopsis
Purpose:
Monitoring tumor response during the course of treatment, and adaptively modifying treatment plan based on tumor biological feedback may represent a new paradigm for radiotherapy1. One recent study has demonstrated the feasibility of tracking the longitudinal tumor apparent diffusion coefficient (ADC) trend throughout the treatment course, which might reflect tumor response, using a 0.35T MRI-guided radiotherapy system (MRIdian, ViewRay, Oakwood Village, OH)2. However, the conventional diffusion-weighted single-shot echo-planar-imaging (DW-ssEPI) technique suffers from limited resolution and severe distortion, which is particularly problematic for radiotherapy. In addition, DW-ssEPI at low field strength becomes inaccurate due to excessive SNR loss. The purpose of this work is to develop a reliable, accurate and distortion-free diffusion sequence that is practicable for longitudinal assessment of tumor response to radiotherapy.Methods:
Sequence: A diffusion-prepared segmented turbo spin echo readout diffusion technique (DP-TSE) was proposed and developed. Twice-refocused spin-echo strategy was used to mitigate eddy currents. At the end of diffusion encoding, the diffusion-encoded transverse magnetization was flipped back to the longitudinal direction, then followed by spoilers to eliminate the remaining transverse magnetization that would violate the Car-Purcell-Meiboom-Gill condition for the subsequent TSE readout. K-space view order was modified such that the central k-space is acquired by the same shot to mitigate the shot inconsistency3.
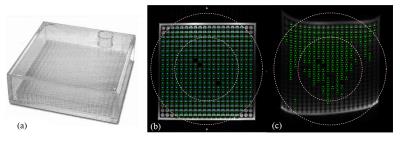
Spatial integrity: A uniformity and linearity phantom (Fluke Biomedical, see Figure 1(a)) was used to assess the spatial integrity under three different orientations: transverse, coronal, and sagittal. The detected cylinder center locations on images were compared with the ground-truth sphere locations.
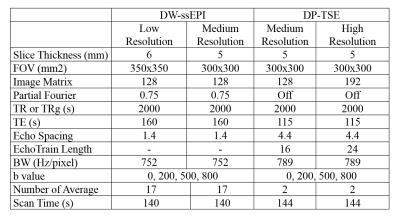
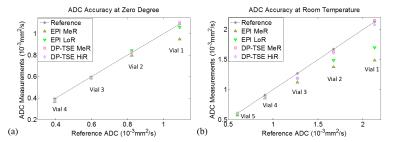
ADC accuracy and reproducibility: ADC accuracy was evaluated using a diffusion phantom (High Precision Device, Inc.) under both 0°C (reference ADCs provided by the vendor) and room temperature to cover a diffusivity range between 0.40×10-3 and 2.10×10-3 mm2/s. Three resolutions (low, medium, and high resolutions) were examined to study the dependence of ADC accuracy on SNR/resolution (see Table 1). To obtain the reference ADCs at room temperature, the phantom was scan at a 3T scanner (Prisma, Siemens Medical Solution) using DW-ssEPI sequence. Ten room temperature measurements on five different days using the high-resolution DP-TSE protocol were conducted to verify the ADC reproducibility which is essential for the robustness of the ADC-based tumor response assessment.
Patient study: Two glioblastoma(GBM) and three sarcoma patients were included to examine the in-vivo feasibility of our DP-TSE technique using the ViewRay system. Quantitative geometric accuracy assessment was evaluated using target registration error(TRE)4, where seven to twelve landmarks were identified by a radiation oncologist on reference images (CT for GBM patients and planning bSSFP MR for sarcoma patients) and diffusion images respectively. For ADC comparison, ROIs were drawn inside the surgical cavity, CSF, and brain tissue in GBM patients, and inside the tumor region in sarcoma patients. For patients not treated on ViewRay, a tube phantom containing diluted gadolinium contrast was placed next to them as a reference (not on patients treated on ViewRay to avoid interference with treatments).
Results and Discussion:
DP-TSE passed the spatial integrity test under all three orientations with a 100% pass rate(Figure 1). The detected errors were 0.474mm±0.355mm, 0.475mm±0.287mm, and 0.546mm±0.336mm in the axial, coronal, and sagittal plane respectively. The spatial integrity test could not be performed for the DW-ssEPI data because the cylinder markers could not be detected in either of the orientation using DW-ssEPI images due to severe distortion and low signal intensity.
ADC accuracy measurements were summarized in Figure 2. At 0°C, ADCs from DP-TSE had an overall of <3% discrepancy with the reference whereas DW-ssEPI could only provide accurate ADC under the low-resolution protocol. At room temperature(21°C), DW-ssEPI failed to provide accurate ADC for high diffusivity vials due to high noise level. Excellent ADC reproducibility with <4% coefficient of variation was observed among the 10 measurements of DP-TSE (temperature=20.68±0.28°C), where the mean ADC for the five vials with different diffusivities were: 2.09±0.03×10-3mm2/s; 1.60±0.02×10-3mm2/s; 1.18±0.02×10-3mm2/s; 0.86±0.02×10-3mm2/s; 0.61±0.02×10-3mm2/s.
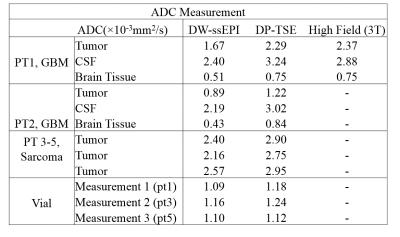
As shown in Figure 3, DP-TSE provided considerably improved geometric accuracy compared to DW-ssEPI. Table 2 is a summary of the ADC measurements on the five patients. For GBM patients, CSF and brain tissue ADCs from DW-ssEPI were below literature range while DP-TSE provided reasonable values. Among the three sarcoma patients, tumor ADCs from DW-ssEPI were ~20% lower than from DP-TSE, consistent with the phantom study where DW-ssEPI underestimated high-diffusivity vials. For the reference tube that had a relatively low diffusivity, the two techniques provided matched measurements.
Conclusion:
A diffusion-prepared TSE-based diffusion technique with excellent geometric fidelity, accurate and highly reproducible ADC measurement was proposed and validated for longitudinal tumor response assessment using an MRI-guided radiotherapy system.Acknowledgements
Yu Gao acknowledges research support from ViewRay.References
[1] H. C. Thoeny and B. D. Ross, “Predicting and Monitoring Cancer Treatment Response with DW-MRI,” J. Magn. Reson. Imaging JMRI, vol. 32, no. 1, pp. 2–16, Jul. 2010.
[2] Y. Yang et al., “Longitudinal diffusion MRI for treatment response assessment: Preliminary experience using an MRI-guided tri-cobalt 60 radiotherapy system,” Med. Phys., vol. 43, no. 3, pp. 1369–1373, Mar. 2016.
[3] M. D. Robson, A. W. Anderson, and J. C. Gore, “Diffusion-weighted multiple shot echo planar imaging of humans without navigation,” Magn. Reson. Med., vol. 38, no. 1, pp. 82–88, Jul. 1997.
[4] C. R. Maurer, J. M. Fitzpatrick, M. Y. Wang, R. L. Galloway, R. J. Maciunas, and G. S. Allen, “Registration of head volume images using implantable fiducial markers,” IEEE Trans. Med. Imaging, vol. 16, no. 4, pp. 447–462, Aug. 1997.
[5] J. Helenius et al., “Diffusion-Weighted MR Imaging in Normal Human Brains in Various Age Groups,” Am. J. Neuroradiol., vol. 23, no. 2, pp. 194–199, Feb. 2002.
[6] R. N. Sener, “Diffusion MRI: apparent diffusion coefficient (ADC) values in the normal brain and a classification of brain disorders based on ADC values,” Comput. Med. Imaging Graph., vol. 25, no. 4, pp. 299–326, Jul. 2001.
Figures