4390
Diffusion Weighted Fast Spin Echo for tumor delineation in head-and-neck radiotherapy: a comparison with FDG-PET1Department of Radiotherapy, UMC Utrecht, Utrecht, Netherlands, 2Department of Radiology, UMC Utrecht, Utrecht, Netherlands, 3Department of Radiation Physics, MD Anderson Cancer Center, TX, United States
Synopsis
DWI shows high contrast between tumor and surrounding tissue, which shows potential for target volume delineation in head-and-neck radiotherapy treatment planning. In this study we assess the performance of a diffusion weighted fast spin echo sequence (DW-SPLICE) for target delineation in terms of interobserver agreement and spatial concordance with PET. Fifteen patients underwent both PET and DW-SPLICE. PET was segmented using a Gaussian mixture model, DW-SPLICE was delineated by 3 observers. Target volume delineation using DWI is feasible in head-and-neck radiotherapy. Using an optimized DWI sequence, target volumes could be defined with good interobserver agreement and large similarity with PET.
Introduction
Diffusion weighted (DW) MRI shows high contrast between tumor and the surrounding tissue, which makes it a candidate to facilitate target volume delineation in head-and-neck (HN) radiotherapy (RT) treatment planning. The acquisition of DW-MRI, using conventional echo planar imaging (EPI), in the HN region suffers from image distortions due to large magnetic field inhomogeneities caused by air-tissue transitions1.
When the EPI readout is replaced with a fast spin echo (FSE) readout, geometrically accurate images can be acquired, allowing the DW images to be used for target delineation2. The introduction of DW gradients can lead to CPMG related problems and unstable echo trains. To address this issue DW-FSE was performed using split acquisition of fast spin-echo signal for diffusion imaging (SPLICE)3.
To assess the performance of this sequence for tumor delineation, 18F-fluorodeoxyglucose
(FDG) positron emission tomography (PET) was used as a reference. PET shows high contrast between tumor and the surrounding tissue and is already used to segment the target automatically4. Additionally, the interobserver variation of target delineation is a valuable tool to evaluate the
utility of the applied sequence.
In this study we assess the performance of DW-SPLICE for target delineation in terms of interobserver agreement and spatial concordance with (semi-)automatic delineation on PET.
Methods
Fifteen HN cancer patient underwent DW-SPLICE in addition
to FDG-PET for RT treatment planning. MR imaging was performed on a 3.0T Philips Ingenia system using 2-element Flex-M surface coils (Philips Healthcare, The Netherlands). Diffusion weighting was applied isotropically in 3 orthogonal directions using b-values 0 and 800 s/mm2. Fat suppression was performed using spectral presaturation with inversion recovery (SPIR). ADC maps were generated directly on the MR system.
Sequence parameters: FOV (RL x AP x FH): 230 x 280 x 120 mm3; Acquired voxel size: 1.8 x 1.8 mm2; Slice thickness: 4 mm; TE/TR: 52/16366 ms; SENSE acceleration factor: 2; Echo train length (dummies): 64 (1); Bandwidth: 900.3; b-values (averages): 0 (2), 800 (5) s/mm2; Total acquisition time: 4m38s.
Target delineation on DW-SPLICE was
performed by 3 observers. First a seed point was placed within the tumor, on the diffusion weighted image (b800), and using an image intensity threshold of 50% of the maximum a region was segmented. After this initial segmentation, the 3 observers adapted these contours individually using the ADC map and the diffusion weighted images (b0 and b800).
For PET, the images were segmented using a Gaussian Mixture Model, which classifies voxels based on differences in their intensity distributions5. This segmentation was initialized by placing a box around the region of the tumor on the PET images.
Volumes, overlap metrics,
defined as dice similarity coefficient (DSC) and generalized
conformity index (CI), and contour distances were calculated from the
delineations. The contour distances are reported as the mean distance (HDmean) and 95th percentile distance (HD95). The results of these metrics are give as the median (range) over all patients.
Results
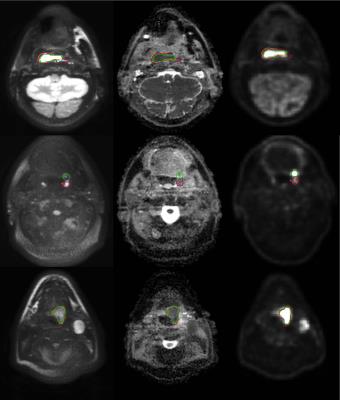
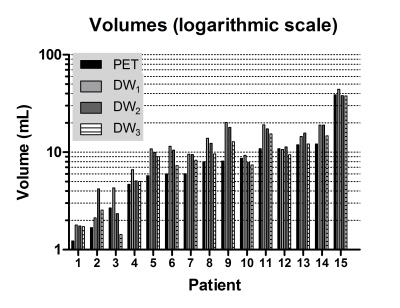
Imaging examples are given in figure 1, showing the correspondence between DWI and PET and also between the different observers on DWI. The median volumes delineated by the 3 observers on DWI were 10.8, 10.5 and 9.0 mL respectively, while on PET the median volume was 8.0 mL, the difference was statistically significant (figure 2).
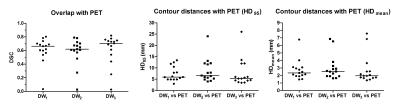
Compared with PET, the delineations by the 3 observers showed a median DSC of 0.71 (0.03-0.80), 0.69 (0.03-0.79) and 0.72 (0.03-0.82) respectively. For all 3 observers, the mean contour distance between DWI and PET was small with median (range) distances of 2.3 (1.5-6.8), 2.5 (1.6-6.9) and 2.0 (1.35-7.6) mm respectively. The median 95th percentile distances were 6.0 (3.0-13.4), 6.6 (4.0-24.0) and 5.3 (3.4-26.0) mm (figure 3).
High interobserver agreement was indicated by a median CI of 0.73 (0.38-0.80) and small distances between the contours of the observer pairs (figure 4).
Discussion / Conclusion
Target volumes could be delineated on DWI with a high interobserver agreement, higher than on conventional MRI6. The lowest agreement (low CI, large contour distances) was found in small tumors adjacent to lymphatic tissue.
The volumes found on DWI were larger than the PET segmentations. However, there was a large overlap between DWI and PET, indicating that they mostly identify the same target for treatment. Differences mainly occur at the edges of the delineated volumes. High DSC's and small contour differences between PET and the respective DWI observers indicate a large similarity between the two modalities.
In conclusion, target volume delineation using DWI is feasible in head-and-neck radiotherapy. Using an optimized DWI sequence, target volumes could be defined with good interobserver agreement and a large similarity with PET.Acknowledgements
Financial support for this work was provided by the Dutch Cancer Society (project UU 2011-5216).References
1. Schakel T, Hoogduin JM, Terhaard CH et al. Diffusion weighted MRI in head-and-neck cancer: geometrical accuracy. Radiother Oncol. 2013;109:394-7.
2. Schakel T, van Yperen GH, van den Brink J et al. Diffusion weighted imaging in head-and-neck cancer: comparison between echo planar and turbo spin echo sequences. In proc ISMRM 2013 #3443
3. Schick F. SPLICE: sub-second diffusion-sensitive MR imaging using a modified fast spin-echo acquisition mode. Magn Reson Med. 1997;38:638-44.
4. Zaidi H, El Naqa I. PET-guided delineation of radiation therapy treatment volumes: a survey of image segmentation techniques. Eur J Nucl Med Mol Imaging. 2010;37:2165-87.
5. Aristophanous M, Penney BC, Martel MK et al. A Gaussian mixture model for definition of lung tumor volumes in positron emission tomography. Med Phys. 2007;34:4223-35
6. Jager EA, Ligtenberg H, Caldas-Magalhaes J et al. Validated guidelines for tumor delineation on magnetic resonance imaging for laryngeal and hypopharyngeal cancer. Acta Oncol. 2016 Nov;55(11):1305-1312.
Figures