4387
Diffusion-weighted MRI of rectal cancer: baseline tumour perfusion fraction predicts chemoradiotherapy response and survival1Department of Oncology, Akershus University Hospital, Lørenskog, Norway, 2Institute of physics, University of Oslo, Oslo, Norway, 3Department of Radiology and Nuclear Medicine, Oslo University Hospital, 4Department of Oncology, Oslo University Hospital, 5Department of Pathology, Oslo University Hospital, 6Department of Tumor Biology, Oslo University Hospital, 7Department of Gastroenterological Surgery, Oslo University Hospital, 8Institute of Clinical Medicine, University of Oslo
Synopsis
More accurate diagnostics for prediction of treatment responses in locally advanced rectal cancer is warranted. We employed a simplified approach to the intravoxel incoherent motion imaging method to estimate the tumour perfusion fraction from diffusion-weighted MRI. The perfusion fraction was predictive of the histologic tumour response after chemoradiotherapy (p = 0.02), and in combination with tumour volume this parameter was also predictive of five-year progression-free survival of the patients (p = 0.002). This simplified approach does not require substantial extra scan time in a routine diagnostic scanning, and may offer a clinically feasible approach to stratifying patients to individualised treatment.
Introduction
Locally
advanced rectal cancer (LARC) is commonly treated with chemoradiotherapy (CRT)
followed by surgery1. Due to heterogeneous treatment responses to
CRT, more accurate diagnostics is needed for improving patient stratification to
treatment intensification, but also for identifying excellent responders for
watch-and-wait approaches. Tumour hypoxia (oxygen deficiency) is acknowledged
as associated with aggressive tumour development and reduced treatment response2,
and this oxygen deficiency is linked to poor perfusion of the tumour. We
employed a simplified approach to the intravoxel incoherent motion (IVIM) method3
to extract both the apparent diffusion coefficient (ADC) and the tumour
perfusion fraction (F) from diffusion-weighted (DW) MRI. The aim was to
investigate whether ADC and F predict histologic tumour regression grade (TRG)
and 5-year progression-free survival (PFS) in LARC. Method
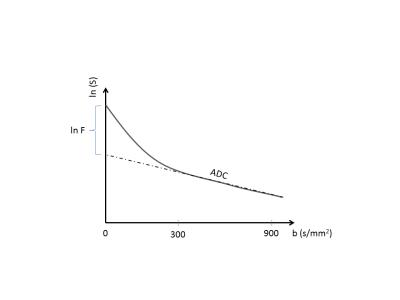
From baseline DWMRI of 27 patients we calculated ADC-maps using b-values
300 and 900 s/mm2. The ADC-values were used to extrapolate a line
asymptotically back to the y-axis, calculating the discrepancy with the
measured signal at b = 0 as F4, Figure 1. We have previously
reported the predictive value of tumour volume for this study5 and we
therefore included volume in our analysis to look for a potentially combined
predictive value. Defining TRG1-2 as good responders and TRG3-5 as poor
responders we used the Mann Whitney U test and receiver operating characteristic
(ROC) curve analysis to investigate each parameters’ predictive value. Five-year
survival was analysed using log rank test and Kaplan Meier plots.Results
ADC, though
higher in good responders (74.1 x 10-4 mm2/s
± 22.7 vs 61.0 ± 12.6, p = 0.13),
was not significantly predictive of TRG, nor of PFS. The perfusion fraction F,
also higher in good responders (22.0 (au) ± 10.9 vs 12.6 ± 4.1, p = 0.02) was
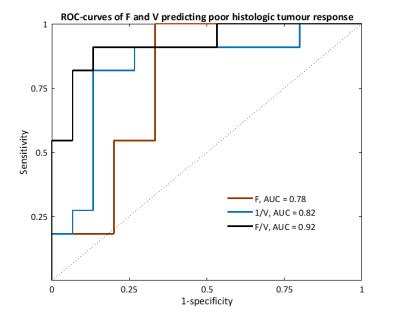
significantly predictive of TRG. The combination of F and volume (F/V) was highly
predictive of TRG (AUC = 0.92, p < 0.001, CI = 0.81–1.00, sensitivity
91%, specificity 87%) and performed better than each parameter separately, Figure 2. F/V
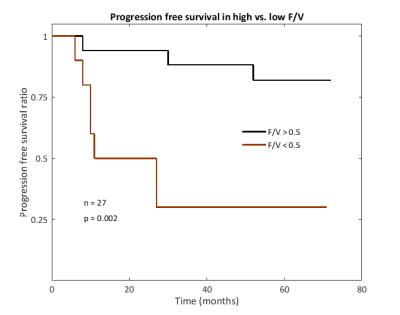
also predicted PFS, with an F/V higher than 0.5 showing a five-year PFS of 82%,
compared to 30% for F/V below 0.5, Figure 3. Discussion
Using a
simplified IVIM approach we identified that baseline tumor F differentiated
good and poor responders to CRT and in combination with tumour volume also
predicted PFS. Low F may be associated with tumour hypoxia and poor perfusion,
and hence radiation resistance. A low perfusion fraction from IVIM-imaging has
previously been associated with poor treatment response in locally advanced
breast cancer6. ADC by itself as a predictive parameter for tumour
response to CRT in LARC has not shown consistent results7, and our study
shows that the additional estimate of the perfusion fraction can have clinical
value. By making a simplified approach to the IVIM method, requiring only three
b-values, the method does not require much extra time or heavy computation, and
may therefore represent a feasible approach for implementation into clinical
routine, and hence, enable improved stratification of LARC patients to individualised
treatment.
Acknowledgements
No acknowledgement found.References
1. GF Weber, R Rosenberg, JE Murphy et al. Multimodal treatment strategies for locally advanced rectal cancer. Expert Rev Anticancer Ther. 2012;12(4);481-94
2. RG Bristow, RP Hill. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer. 2008;8(3):180-92
3. D Le Bihan, E Breton, D Lallemand et al. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168(2);497-505
4. R Wirestam, M Borg, S Brockstedt et al. Perfusion-related parameters in intravoxel incoherent motion MR imaging compared with CBV and CBF measured by dynamic susceptibility-contrast MR technique. Acta Radiol. 2001;42(2);123-8
5. T Seierstad, KH Hole, KK Grøholt et al. MRI volumetry for prediction of tumour response to neoadjuvant chemotherapy followed by chemoradiotherapy in locally advanced rectal cancer. BR J Radiol. 2015;88(1051)20150097
6. S Che, X Zhao, Y OU et al. Role of the Intravoxel Incoherent Motion Diffusion Weighted Imaging in the Pre-treatment Prediction and Early Response Monitoring to Neoadjuvant Chemotherapy in Locally Advanced Breast Cancer. Medicine. 2016;95(4);e2420
7. RGH Beets-Tan, GL Beets. MRI for assessing and predicting response to neoadjuvant treatment in rectal cancer. Nat. Rev. Gastroenterol. Hepatol. 2014;11;480-488
Figures