4368
In-vivo Detection of Acute Intracellular Acidification in Glioblastoma Multiforme by AACID CEST MRI Following a Single Dose of Cariporide and Quercetin1Department of Medical Biophysics, University of Western Ontario, London, ON, Canada, 2The Centre for Functional and Metabolic Mapping, Robarts Research Institute, University of Western Ontario, London, ON, Canada, 3Department of Biochemistry, University of Western Ontario, London, ON, Canada
Synopsis
Identification of tumor boundaries is challenging due to the infiltrative nature of
Purpose:
Glioblastoma Multiforme (GBM) remains one of the most lethal cancers with a mean survival of 12-18 months. Clear identification of tumor boundaries is challenging due to the infiltrative nature of the cancer. In this work we explore the use of a physiological challenge to detect cancer cells. We have previously shown that both lonidamine and topiramate can selectively acidify GBM tumors. Other drugs approved for human use can target different pH regulatory mechanisms. For example, cariporide is a Na⁺/H⁺ exchange inhibitor and quercetin is a monocarboxylate transport inhibitor. The goal of the current study is to determine whether chemical exchange saturation transfer (CEST) MRI is sensitive to tumor acidification after cariporide or quercetin injection. We hypothesized that both cariporide and quercetin would selectively increase tumor acidity within 2 hours of injection.Methods:
CEST is a relatively novel MRI contrast mechanism that can be made to be dependent on intracellular pH (pHi). Amine and amide concentration-independent detection (AACID)1,2 is a recently developed CEST contrast that is sensitive to intracellular acidification. AACID is proportional to the ratio of the amine CEST effect observed at 2.75 ppm and the amide CEST effect at 3.5 ppm and is inversely related to pHi. CEST images (2 averages) were acquired on a 9.4T Agilent (Santa Clara, CA) MRI scanner using a fast spin-echo (FSE) pulse sequence1,2 (TR/TE = 7000/7 ms, ETL = 32, effective TE = 7 ms, FOV = 25.6 x 25.6 mm2, matrix size = 64 x 64, slice thickness = 2 mm) preceded by a continuous RF pulse with amplitude 1.5-µT and duration 4-seconds. The CEST images were acquired at different saturation frequencies (1.2 to 4.5 (∆=0.1) ppm, from 5.4 to 6.6 (∆=0.1) ppm, -1000 and 1000 ppm images were acquired as a reference, total 49 images). CEST images were acquired on all mice approximately 14 days after implanting 105 U87 human glioblastoma multiforme cells in the brain, before and immediately after administration of cariporide (dose: 6 mg/kg, N=6)3 or quercetin (dose: 200mg/kg, N=6). Both cariporide and quercetin were dissolved in dimethyl sulfoxide (DMSO). Cariporide was diluted with phosphate buffered saline (PBS) then injected intraperitoneal (i.p.) over 2 min until the dose was achieved. Three mice were also used as controls in each study to assess the effect of the vehicle alone on pHi. The MR imaging protocol also included anatomical T1-, T2-, and diffusion-weighted imaging. For B0 correction, the water saturation shift referencing (WASSR) technique was used.5 CEST images were reacquired ~2 hours following cariporide or quercetin injection. AACID maps were produced1,2 to measure AACID values in all mice in manually defined regions of interest (ROIs) containing tumor and contralateral tissue. All animals were sacrificed post imaging.Results:
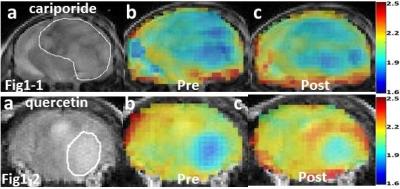
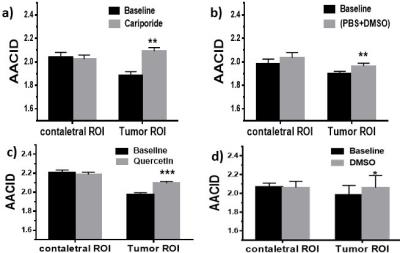
Figure 1-1a and Figure 1-2a show T2-weighted images of the brain tumor in two different mice. AACID maps prior to injection (Figures1-1b,1-2b) show decreased AACID values (higher pHi) in the tumor compared to surrounding brain tissue as expected. Immediately after drug injection an increase in AACID value is observed in the tumors (Figures1-1c,1-2c). Figure 2a shows the average changes in AACID values for the six mice receiving cariporide. In the tumor ROI the AACID value significantly increased after cariporide injection by 0.21±0.02 (p< 0.05), but there was no average change in AACID within the contralateral ROI. There was also a small but significant change in AACID in the tumor for the three mice injected with PBS+DMSO only (control, Figure 2b). Figure 2c shows the changes in AACID for the six mice receiving quercetin. In the tumor ROI the AACID value significantly increased after quercetin injection by 0.12±0.03 (p< 0.005), but there was no change in AACID within the contralateral ROI. Again there was a small but significant change in AACID for three mice injected with DMSO only (control, Figure 2d).Discussion:
In this experiment we used cariporide and quercetin to induce an acute metabolic change detectable by endogenous MRI CEST4 contrast. The observed increase in AACID value corresponds to a decrease in pHi within the tumor and is likely due to the blockage of the Na⁺/H⁺ exchanger in the case of cariporide or blockage of monocarboxylate transporters in the case of quercetin. Cariporide is approved in humans, while quercetin is a natural compound. DMSO also appears to have a small but positive effect on tumor acidification.The physiological change induced by cariporide or quercetin in combination with DMSO could help localize brain cancer and monitor tumour response to chemotherapy. This unique approach to cancer detection does not require injection of an imaging contrast agent.Acknowledgements
We would like to thank Miranda Bellyou and Ashley Kirley for assistance with animal handling. Funding provided by the Ontario Institute for Cancer Research (OICR).References
1. McVicar, N., Li, A.X., Meakin, S.O. and Bartha, R., 2015. Imaging chemical exchange saturation transfer (CEST) effects following tumor-selective acidification using lonidamine. NMR in biomedicine, 28(5), pp.566-575.
2. Marathe, K., McVicar, N., Li, A., Bellyou, M., Meakin, S. and Bartha, R., 2016. Topiramate induces acute intracellular acidification in glioblastoma. Journal of Neuro-Oncology, pp.1-8.
3. Harguindey, S., Arranz, J.L., Orozco, J.D.P., Rauch, C., Fais, S., Cardone, R.A. and Reshkin, S.J., 2013. Cariporide and other new and powerful NHE1 inhibitors as potentially selective anticancer drugs–an integral molecular/biochemical/metabolic/clinical approach after one hundred years of cancer research. Journal of translational medicine, 11(1), p.1.
4. Sagiyama, K., Mashimo, T., Togao, O., Vemireddy, V., Hatanpaa, K.J., Maher, E.A., Mickey, B.E., Pan, E., Sherry, A.D., Bachoo, R.M. and Takahashi, M., 2014. In vivo chemical exchange saturation transfer imaging allows early detection of a therapeutic response in glioblastoma. Proceedings of the National Academy of Sciences, 111(12), pp.4542-4547.
5. Kim, M., Gillen, J., Landman, B.A., Zhou, J. and van Zijl, P., 2009. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magnetic resonance in medicine, 61(6), pp.1441-1450.
Figures