4363
Impacting cancer cells via mechanical waves: can we change cellular behaviour?1Biomedical Engineering, King's College London, London, United Kingdom, 2Tissue Engineering & Biophotonics, King's College London, 3Imaging Chemistry and Biology, King's College London, London, United Kingdom, 4Biomedical Engineering, King's College London, Lodon, United Kingdom
Synopsis
90% of cancer related deaths are caused by cancer metastasis, a process where cells leave the primary tumour, disseminate and form outgrowth at the secondary metastatic site. The tumour microenvironment provides crucial signals ((bio)chemical, mechanical) to regulate tumour formation, progression, and cell spread throughout the body. Translation of mechanical forces, displacements and deformations into biochemical signals (i.e. mechanotransduction) affects their cell behaviour (adhesion, spread, survival).1,2 We show here that multiple treatment of tumour spheroids (solid tumour model in vitro) with focussed shear waves operating at specific frequency and amplitude results in reduced growth and reduced invasive behaviour of cancer cells.
Purpose
To evaluate a non-invasive method which alters cancer cell motility by inducing focused shear waves at specific frequencies and amplitudes multiple times to follow a therapeutic approach.Introduction
In the last decade cancer became a major public health problem as 1 in 2 people will be diagnosed with cancer and every 4th will die from it. The majority of cancer deaths are caused by cancer metastasis, where cancer cells leave the primary tumour and form outgrowths at a secondary site. Microenvironmental signals of (bio)chemical and mechanical nature play a key role in this process.3,4 Work has shown that mechanical forces (stresses), displacements (shear) and deformations can translate into biochemical signals affecting cancer cell survival. Here we study a novel approach of utilizing oscillatory low frequency shear waves to non-invasively impact cancer growth and invasive behaviour. We validate the method in vitro, with the aim of investigating in vivo the changes in metastatic potential.Material & Methods
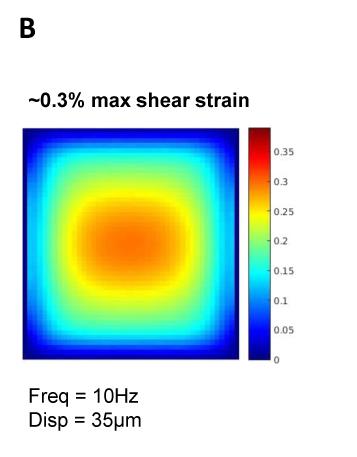
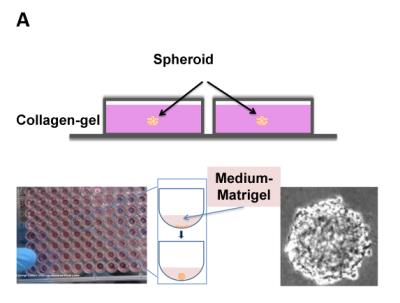
A metastatic human breast cancer cell line, MDA-MB 231, was used for all experiments. By forming tumour spheroids embedded into a collagen matrix (Image A), we create a 3D microenvironment. This experimental set-up models the behaviour and cell signalling of a solid tumour in a human body and therefore creates an in vivo-like environment within an in vitro model. To investigate the influence of focussed shear waves on cancer cells, tumour spheroids were formed and exposed to multiple shaking treatments lasting 3h/day for 4 days at 10 Hz, 35µm displacement. Spheroids were monitored over 5 days by taking pictures with a microscope. Magnetic resonance electrography (MRE) was used to characterise the stiffness of the bovine collagen type 1 matrix to simulate the area of maximum shear stress within the well (Image B). To prove that the integrity and mechanical shear properties of the gel do not change as a consequence of the mechanical shaking, MRE and second harmonic microscopy (SHG) were performed.Results
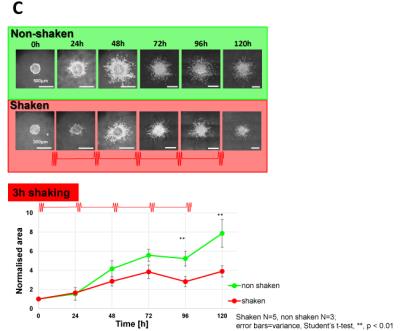
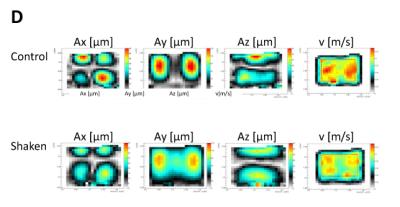
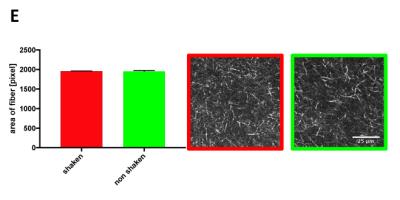
Image C shows in green the “baseline” growth and invasion of a non-shaken spheroid in the matrix and in red the shaken spheroid. Statistical analysis of the pictures shows a reduction of growth and invasion of the shaken spheroid in comparison to the non-shaken spheroid. The first noticeable difference is seen after 48h and increases over time. After 5 days the treatment reduces the growth/invasion by a factor of 2. To ensure that shaking does not affect the structure of the collagen, shaken vs non-shaken samples were analysed via MRE (Image D) and SHG (Image E). Both methods show that the gel does not undergo any changes during or after the shaking process.Discussion & Conclusion
The experiments showed that multiple induction of shear stress diminishes tumour spheroids’ ability of growing and invading into the collagen. Even more, they indicate that the cells are not able to adjust to the treatment, as multiple treatments further impede the growth and invasion. Proving that shaking causes no structural changes within the collagen was important for validating the results, as changes of the extra-cellular matrix could trigger changes in the cellular behaviour. In conclusion we showed that exposing a tumour spheroid multiple times to low frequency shear strain inhibits growth and invasive behaviour by a factor of 2. The fact that cancer cells do not adjust to the treatment would enable a future therapeutic approach of this technique. As such, MRE could be used not only as a monitoring tool, but also as part of a therapeutic treatment.Acknowledgements
This research was funded/supported by Cancer Research UK, Cancer Imaging Centre, Medical Engineering Initiative, EPSRC Pioneering research and skills, Medical Research Council and Department of Health. This research was funded/supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London
European Union’s Horizon 2020 research and innovation programme under grant agreement No 668039. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
References
1.Valastyan, Cell 2011, October 14
2.Barney, Chemical Engineering 2016, 11:85–93
3.Cheng, PlosOne2009;4(2):e4632
4.Polacheck, PNAS 2014;11(7) no. 72447–2452
Figures
(A)Set-up of the embedded spheroid (side view), spheroid formation via spinning method.