4361
in vivo detection of tumor response to radiotherapy using Imaging Micro-structural Parameters Using Limited Spectrally Edited Diffsuion1vanderbilt university institute of imaging science, nashville, TN, United States
Synopsis
The changes that occur over a cell cycle play a vital role in mediating a cell’s sensitivity towards radiation therapy. Radiation exposure is expected to arrest cells at a particular cell cycle phase which improves the effectiveness of subsequent doses of radiation/chemotherapy. Cells in different phases have different sizes that can be detected by diffusion MRI with appropriate diffusion times. In this study, we evaluate the hypothesis in a rat glioma model that measurements of mean tumor cell size provides a means to quantify changes of cell phase distributions, and hence is capable of monitoring tumor response to radiotherapy.
Background
The changes that occur over a cell cycle play a vital role in mediating a cell’s sensitivity towards radiation therapy. Radiation exposure itself can alter cell cycle dynamics through the activation of various intracellular pathways. For example, radiation exposure is expected to synchronize cell populations and arrest cells at a particular cell cycle phase which improves the effectiveness of subsequent doses of radiation or chemotherapy. Therefore, monitoring cell cycle dynamics is important to optimize treatment protocols that improve survival rates. Cells in different phases of the cell cycle have been reported to have different sizes that can be detected by diffusion MRI with appropriate diffusion times1. We hypothesize that measurements of mean tumor cell size provides a unique means to quantify changes of cell phase distributions, and hence are capable of monitoring tumor response to radiation treatment. Recently, we reported a new in vivo approach, termed IMPULSED (Imaging Microstructural Parameters Using Limited Spectrally Edited Diffusion) to accurately quantify mean cell sizes using temporal diffusion spectroscopy with diffusion-weighted acquisitions over a broad range of diffusion times2. In this study, we evaluate the feasibility of this method to characterize the radiation treatment response of tumors, especially the tumor cell size changes associated with cell cycle progression in a rat glioma model.Theory and Methods
Theory: The diffusion weighted signals from tissues are assumed to be the sum of signals arising from intra- and extracellular spaces, namely, S=Vin*Sin+(1-Vin)*Sex, where Vin is the water volume fraction of intracellular space, and Sin and Sex are the signal magnitudes per volume from the intra- and extracellular spaces, respectively. Cells are modeled as impermeable spheres of diameter d. The analytical expression for the diffusion weighted signal for water within an impermeable sphere of size d and intrinsic diffusion rate Din has been reported previously and was assumed here. Sex=exp[−𝑏(Dex0+𝛽ex∙𝑓)], where Dex0 is the extracellular diffusion rate at frequencies close to 0, and βex is the slope of extracellular diffusion coefficient with respect to frequency f, which also contains information on structural dimensions.
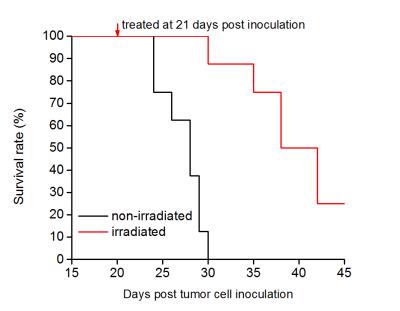
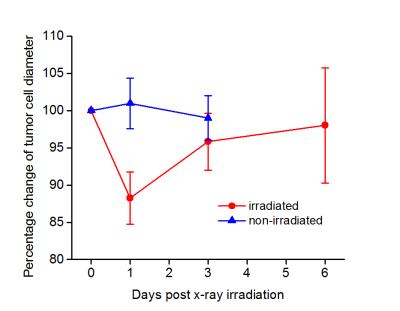
In vivo experiments: A rat glioma cell line (9L) was used to create tumors in a total of 30 rats. Half of 9L-bearing rats were treated with a single fraction of 20 Gy of x-ray radiation. Of all the 9L-bearing rats, 16 (8 irradiated and 8 non-irradiated) were used for survival studies, and 14 (8 irradiated and 6 non-irradiated) for the characterization of radiation treatment response using the IMPULSED method. Diffusion-weighted images covering the tumor region were collected before, and 1, 3, and 6 days post irradiation. A region of temporal diffusion spectra with diffusion times ranging from ≈ 3 to 48 ms were obtained at 9.4 T using a combination of PGSE and OGSE acquisitions.
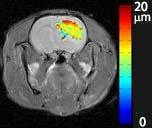
Data analysis: The survivals of animals were represented on Kaplan-Meier curves. The diffusion MR signals were fit to equations reported in our previous publication2 on a voxel-wise basis to derive parametric images of the cell size d.