4354
Using MRI to investigate the impact of radiation-induced damage on promoting tumor growth in a mouse model of brain metastasisAmanda M Hamilton1, Eugene Wong2, and Paula J Foster1,2
1Imaging Research Laboratories, Robarts Research Institute, London, ON, Canada, 2Medical Biophysics, University of Western Ontario, London, ON, Canada
Synopsis
Whole brain radiotherapy (RT) is the standard of care for breast cancer patients with multiple brain metastases but there are multiple negative consequences associated with the irradiation of normal brain tissue. In our study we investigated the influence that RT-induced damage of healthy brain has on the arrest and growth of metastatic breast cancer cells in a mouse model of breast cancer brain metastasis. We observed that irradiated but otherwise healthy neural tissue had an increased propensity to support metastatic growth compared to control. Elucidating the impact of RT on normal neural tissue could have implications in clinical patient management.
Introduction
Whole brain radiotherapy (RT) is the standard of care for breast cancer patients with multiple brain metastases. While this treatment has been shown essential to the management of existing brain tumors, RT is known to have multiple negative consequences in normal brain tissue including radio-necrosis, cognitive deficits and both short and long term inflammation.1 Several studies have also suggested that RT of normal tissues may promote the invasiveness of cancer cells. For example, Bouchard et al. showed that RT of normal mouse mammary tissue induced migration from a contralateral mammary tumor, increased the number of circulating cancer cells and the incidence of lung metastases.2 In our study we built on these findings to investigate the influence that RT-induced inflammation in the healthy brain has on the arrest and growth of metastatic breast cancer cells in a model of breast cancer brain metastasis.Methods
Seven days before cell delivery (day -7) our experimental (RT) female BALB/c mice (Charles River Laboratories, n=4) received 10Gy WBRT in one fraction. Control mice (n=3) were not irradiated. Murine 4T1-BR5 mammary carcinoma cells were labeled with 25 μg Fe/mL MPIO beads (0.9 μm, Bangs Laboratory) in complete DMEM media for 24h. 2x104 MPIO-labeled cells were injected into the left ventricle of anesthetized mice by ultrasound guidance. All animals were imaged on a 3T GE Discovery MR750 whole-body clinical MR scanner using a custom-built high performance gradient coil with a solenoid radio-frequency mouse head coil and a 3D balanced steady-state free precession (bSSFP) sequence. Mice were imaged for proof of cell delivery on day 0 using the following parameters: resolution: 100x100x200 μm3, TR/TE = 8/4ms, flip angle = 35o, bandwidth = ± 42kHz, 4 phase cycles, scan time = 14 min. Mice were imaged for tumor assessment on day 13 with the same parameters as above except: TR/TE = 10/5ms, bandwidth = ±12.5kHz, 8 phase cycles, NEX = 2, scan time = 36 min. Images were analyzed using OsiriX image software and assessed for tumor number, total tumor burden and average tumor volume per mouse brain. Statistical analysis was performed using GraphPad Prism V7.0 and assessed using a 2-tailed student t test. After end point imaging mice were sacrificed, perfusion fixed and brains were excised for histological assessment.Results
Imaging mice on the day of MPIO-labeled cell injection using our validated single-cell protocol3 permitted the quantification of cell delivery to the brain (Figure 1A&B, white arrowheads = voids from single iron labeled cells). There was no significant difference in the number of detected signal voids in control mice compared to RT mice (p=0.8686, Figure 1C). At endpoint (day 13), metastases appeared in bSSFP images as high signal intensity regions compared to normal brain parenchyma (Figure 2A&B, black arrows = metastases). Image analysis revealed a significant difference in the observed number (p=0.0039, Figure 2C) of detectable brain tumors with 34±8 and 58±4 metastases observed in the control versus RT groups, respectively. The RT group also displayed a significantly greater average tumor volume (0.24±0.03 mm3) compared the control mouse brains (0.10±0.01 mm3, p=0.0014, Figure 2D). Subsequently these two distinct differences in tumor progression resulted in a very significant difference (p=0.0007, Figure 2E) in total tumor burden between experimental groups (control = 3.49±0.89 mm3, RT = 13.79±2,27 mm3).Discussion
Elucidating the impact of RT on normal neural tissue could have implications in the management of patient treatment. We observed no significant difference in the number of signal voids detected in the brains of each mouse group, therefore there was no evidence that irradiation of normal tissue has any effect on cancer cell arrest. End point data, however, clearly showed that neural tissue that had been irradiated but was otherwise healthy had an increased propensity to support metastatic tumor growth. This was evident by the increased number, average volume and total burden of tumors in the irradiated mouse brain thereby demonstrating that as a result of whole brain RT cancer cells were able to form tumors with greater efficiency (increased number) and at a greater rate (increased volume and total burden) than in normal neural tissue. This preclinical data suggests that there may be an increased risk of recurrence particularly in patients with residual systemic disease or with residual radio-resistant brain cancer. Our next research step will include the assessment of potential adjunct therapies to mitigate the increased risk of metastasis progression in irradiated normal brain.Acknowledgements
Authors would like to thank the Brain Tumour Foundation of Canada for funding supportReferences
1) Moravan MJ, Olschowka JA, Williams JP, O’Banion MK. Cranial irradiation leads to acute and persistent neuroinflammation with delayed increases in T-cell infiltration and CD11c expression in C57Bl/6 mouse brain. Radiat Res. 2011;176(4):459-473. 2) Bouchard G, Bouvette G, Therriault H, et al. Pre-irradiation of mouse mammary gland stimulates cancer cell migration and development of lung metastases. Br J Cancer. 2013;109:1829-1838. 3) Heyn C, Ronald JA, Mackenzie LT, et al. In vivo magnetic resonance imaging of single cells in mouse brain with optical validation. Magn Reson Med. 2006;55(1):23-29.Figures
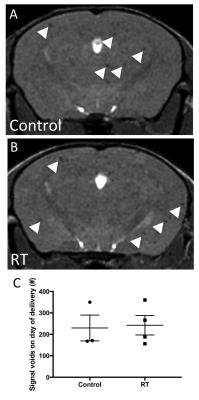
Figure 1. Comparison
of cell delivery between control and RT mice on the day of injection. Individual MPIO-labeled 4T1-BR5 cells
appeared as distinct signal voids (white arrowheads) in bSSFP MR images of
control (A) and RT (B) mouse brains.
There was no significant difference in the arrest of cancer cells
between experimental groups on the day of cell delivery (C).
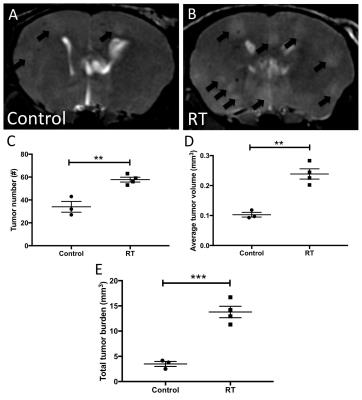
Figure 2. At end
point tumor progression differed in control and RT groups. Representative bSSFP
MR images of control (A) and RT (B) mouse brains are shown with tumors indicated by
black arrows. Significantly greater
number of tumors
(C), average tumor volume (D) and total tumor
burden (E) were observed in RT mouse brains compared to non-irradiated control brains.