4345
Evaluation of an Anthropomorphic Phantom with In-Vivo Using Quantitative MRI1Bioengineering, University of Pittsburgh, Pittsburgh, PA, United States, 2Radiology, University of Pittsburgh, PA, United States
Synopsis
In this work, a developed refillable multi-compartment 3D-printed head phantom (established from MRI scans obtained in-vivo) was compared to a homogeneous commercial spherical phantom, the phantom itself with homogeneous loading in all of its compartments and in-vivo (the same volunteer on whom the phantom was based). Through B1 mapping and SAR analysis within an RF coil, the heterogeneous multi-compartment head phantom results were most accurate to the in-vivo volunteer.
Purpose
Experimental phantom studies lack the accuracy of comparison to in-vivo humans. The purpose of this work is to evaluate the performance and characterization of a physical (3D printed) anthropomorphic heterogeneous head phantom using an RF head coil through simulations of B1 fields and SAR distributions and experiments (in-vivo and phantom B1 field mapping). The designed phantom was compared to a homogeneous commercial spherical phantom, the phantom itself with homogeneous loading (saline) in all of its compartments and in-vivo (the same volunteer on whom the phantom was based).Methods
Fabrication. Wood et. al established an eight-tissue multi-compartment heterogeneous head phantom developed from a high resolution 3T MRI dataset of a healthy male volunteer for various electromagnetic applications 1. The dielectric properties of the heterogeneous head phantom were modeled from Table 1 in 1. Unlike 2, hydrophobic SLA resin is used to hold the liquids of each refillable designed tissue chamber.
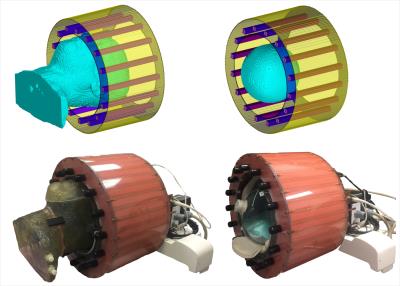
Numerical Modeling. In-house numerical simulation software was used to generate magnetic and electric fields at 297.2 MHz (7T). Numerical simulations are performed on the model of the anthropomorphic heterogeneous head phantom (with the plastic), a commercial homogeneous spherical phantom and the model based on the actual segmentation (no plastic) of the in-vivo images within a 16-strut TEM resonator using finite difference time domain method (FDTD) (Fig 1.)
B1 Mapping. Experimental studies are performed using 7T Siemens MAGNETOM® scanner (Siemens, Erlangen, Germany). To quantify the magnetic field through experimentation, we use SAT Turbo flash (SatTFL) sequence for B1 mapping method, with a rectangular RF pulse of 1 ms at 500V. The MR protocol uses the following image parameters: FOV: 64 x 64 mm2; TE: 1.16 ms; TR: 2000 ms; FA: 6⁰; BW: 1502 MHz; Resolution: 3.125 x 3.125 x 2.0 mm3.
Sample Size. One healthy adult human subject was scanned using the 7T scanner for the in-vivo experimental study with proper consent and IRB approval.
Results
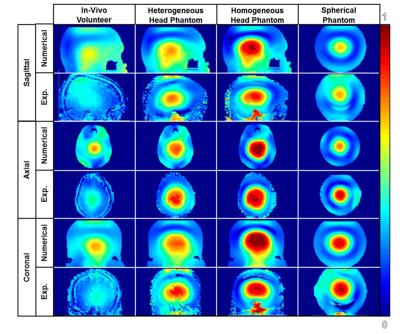
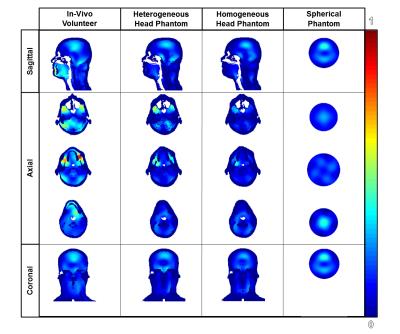
Fig 2 highlights the distributions of the normalized B1+ magnetic field with respect to various planes of view through numerical and experimental studies. The normalized B1 mean values are 1.483uT, 1.545uT, 1.576uT and 1.101uT for the 4 different phantoms. Fig 3 highlights the SAR of the model of each phantom. The calculations for the peak SAR of the various models are 15.2232 W/kg/10g, 12.3558 W/kg/10g, 6.56 W/kg/10g and 6.73 W/kg/10g, according to the order that the images in Fig 3 from left to right.Discussion
Fig 2 depicts the similarities and differences of the normalized B1 magnetic field distribution in the various phantoms compared to the in-vivo volunteer. According to B1 profiles of the experimental data, the magnetic field distribution of the heterogeneous multi-compartment head phantom is most similar to the in-vivo distribution. Although the same phantom was used with homogeneous loading, the magnetic field distribution proved to be most similar to the spherical phantom. This finding indicates that the dielectric properties and complexity of the head phantom have a great role in the accuracy of the phantom’s comparison to the in-vivo volunteer. Fig 3 highlights the distribution of the SAR using the numerical models scaled by the maximum peak SAR among all models. The peak SAR is the greatest in the numerical model of the in-vivo volunteer followed by the heterogeneous phantom. The absence of SAR in the averaged bone/fat/skin tissue or plastic leads to the difference among the four models. The homogeneous multi-compartment phantom is similar in terms of peak SAR value to the spherical phantom.Conclusion
The experiment supports the initial statement being that a commercial spherical phantom or homogeneous multi-compartment phantom lacks accuracy of comparison to in-vivo. While the heterogeneous head phantom is still an estimation, our work demonstrates that the use of a heterogeneous head phantom provides a greater estimation of the electromagnetic behavior (B1 and SAR) occurred in-vivo. Future work should be completed to evaluate the temperature rise in the different compartments of the phantoms and compared to temperature calculations.Acknowledgements
The work reported in this abstract is supported by National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under award number 1F31EB019872. (https://projectreporter.nih.gov/project_info_details.cfm?icde=0&aid=8838518)
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (https://www.nibib.nih.gov/).
References
1. Wood S, Krishnamurthy N, Zhao Y, et al.: Anatomically Detailed Human Head Phantom for MR Testing Purposes. In Jt Annu Meet ISMRM-ESMRMB 2014; 2013:3067.
2. Grädel NN, Polimeni JR, Guerin B, Gagoski B, Wald LL: An Anatomically Realistic Temperature Phantom of the Head for Validation of {SAR} Calculations {[Abstract]}. Proc Int Soc Magn Reson Med 2012; 20:314.
Figures