4341
High field MRI in-vivo setup for observing brain plasticity in Eastern Fenced Lizards1PSU, University Park, PA, United States, 2HUCK Institute, PSU, University Park, PA, United States, 3PSU, Altoona, PA, United States
Synopsis
In this work, Eastern Fenced lizards, a non-model species known for high neurogenesis, were used to explore the extent of adult brain plasticity after exposing the animals to a complex environment and inmates. To conduct this study a MR setup for in-vivo lizard imaging was designed, constructed and tested. High resolution baseline in-vivo three dimensional lizard brain MR microscopy data sets were acquired and analyzed. The high quality of the images will allow to detect any brain volume changes after exposing the lizards to the enriched environment and having them scanned a second time.
Purpose
Using Magnetic Resonance Imaging (MRI) allows non-invasive observation on both physiological and anatomical information of living organisms. Non-invasiveness and high soft tissue contrast make MRI an indispensable tool in clinical research. Traditionally, it was believed that the adult brain is fixed. Although the question of neurogenesis and brain plasticity raised by Joseph Altman in 1960s was rejected by the scientific community, recent neural studies show proliferation of new neurons in adult brain when exposed to external stimulus.[1] It was also verified that enriched environment, which involves complex constructions, induces high neurogenesis rates.[2] It was proven reptiles exhibit much higher brain plasticity, remodeling, and repair rates after injury compared to mammals. [3] Thus, the purposes of this study was to build a custom High-Field MRI setup (14.1tesla, Agilent micro imager) to image the brain of adult lizards in vivo for the first time and to investigate brain plasticity in the telencephalon of lizards after exposing them to enriched environments.Method
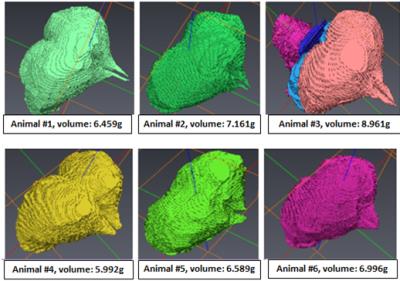
Due to the high sensitivity of single loop surface RF-resonators for local imaging, a single loop surface resonator with an inner diameter of 10mm was designed and built for the in-vivo lizard MRI (Figure 1.a). Compared to rodents, lizards do not have incisors and therefore, a standard rodent animal holding system with biting bar could not be used. Furthermore, special care had to be taken when anaesthetizing the lizard as they can hold their breath for 15-20s regularly. In order to efficiently anesthetize and minimize movements of the lizard to avoid motion artifacts, a newly designed lizard head holder (Figure 1.b) and the body holder (Figure1. c) were constructed considering the size and the anatomical structures of the lizards. To verify the performance of the resonator, B1 mapping [4] was conducted with a 1% Magnevist (Bayer, Germany)-distilled water solution. MatLab (The MathWorks, USA) was used to generate the B1 map, and the result was compared with electromagnetic field simulations conducted in XFdtd (Remcom, USA). Eight Eastern Fence lizards were used for the project. For anesthesia, isoflurane was used and the breathing rate was monitored with a small animal monitoring system (SAII, USA). A standard gradient echo sequence with an echo time (TE) of 3.5ms, a repetition time (TR) of 30ms, a flip angle of 20-degrees, and an isotropic resolution of 50-mictrometer was used to achieve a high resolution 3 dimensional dataset of the lizard brain. These obtained baseline MR images were reconstructed using MatLab (zero filling factor of 2), and the telencephalons were segmented using Avizo (FEI, USA). After the segmentation, the volumes of the telencephalon were calculated, and the lizards were introduced to enriched environments.Result
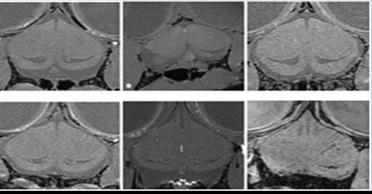
The performance of the custom-built high-field lizard MR setup was tested on a phantom (Figure 2a) and a B1 map was required. The result agreed with the XFdtd simulation very well and showed the typical surface coil B1 distribution (Figure 2b). In-vivo lizard brain baselines were obtained using the MR setup. The acquired images were of high quality and did not show any artifacts on the telencephalon (Figure 3). Thus the results verified high functionality of both surface coil and the animal holder. The obtained images were reconstructed and the baseline volume of the telencephalon was determined (Figure 4).Discussion
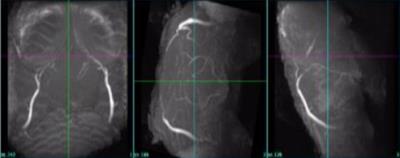
So far, to the best of our knowledge, in-vivo MRI scanning focusing on the brain of non-model species, such as lizards, has not been performed. MR images obtained using the constructed MR setup display high resolution and contrast which allows clear detection of the telencephalon. A good indicator for motion free imaging was an additional maximum intensity projection reconstruction that could be performed. This time of flight reconstruction showed major vessels, such as carotids and arteries, and small cerebral vessels in the lizard brain (figure 5). The obtained lizard brain MR data will be the baseline for this project. The comparison between data before and after the lizards are being exposed to enrich environments will be performed in the next weeks. The outcome from these comparisons made on the lizard telencephalons’ volumetric change can be used as a useful model of brain injuries and repair. Overall, we constructed a MR setup for a non-model species that provided high resolution, high quality in vivo MR images and can be further used in other in-vivo non-model species like fishes or other reptile species.[5]Acknowledgements
I would like to express great gratitude to my devoted parents, Han-Soo Kim and Kyoung-Young Kim, who have been my biggest supporters throughout my whole life. Mom, dad, without your devotion and love, I would never be able to become a person who I am! I am always so thankful and grateful to have you as my parents. If I were given another chance to live my life a second time, I would not even hesitate a second to choose both of you as my parents again.I would also love to thank my two lovely, precious sisters who always cheer me up and make me laugh. Sunny and Esther, thank you so much for being my sisters and my best friends! Just as you girls are always supporting me, I am right by your side, and always will be! I thank both Dr. Thomas Neuberger and Ms. Tatjana Neuberger. Dr. Neuberger, I owe you a debt of gratitude for helping me widen my knowledgeof MRI and engineering in general, and also guiding me throughout my research project! Ms. Neuberger, thank you so much for all the sincere advice and stories you’ve shared with me. I will always keep the lessons that I have learned from our conversations deep in my heart! Finally, I wish best luck to Gangchae, who helped me alot whenever I faced with complications with my research.References
[1] C. G. Gross, “Three before their time: Neuroscientists whose ideas were ignored by their contemporaries,” in Experimental Brain Research, 2009, vol. 192, no. 3, pp. 321–334.
[2] H. van Praag, G. Kempermann, and F. H. Gage, “Neural consequences of environmental enrichment,” Nat Rev Neurosci, vol. 1, no. 3, pp. 191–198, 2000.
[3] E. Font, E. Desfilis, M. Pérez-Cañellas, S. Alcantara, and J. M. García-Verdugo, “3-Acetylpyridine-induced degeneration and regeneration in the adult lizard brain: A qualitative and quantitative analysis,” Brain Res., vol. 754, no. 1–2, pp. 245–259, 1997.
[4] S. L. Talagala and J. Gillen, “Experimental determination of three-dimensional RF magnetic field distribution of nmr coils,” J. Magn. Reson., vol. 94, no. 3, pp. 493–500, 1991.
[5] C. Maximino et al., “Non-mammalian models in behavioral neuroscience: consequences for biological psychiatry,” Front. Behav. Neurosci., vol. 9, no. 233, p. 21, 2015.
Figures
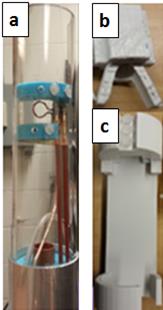
Figure 1: (a) 10mm single loop surface coil for in-vivo lizard brain scanning.
(b) Lizard head holder including isoflurane delivery chamber for minimizing the head movement. This part is critical as lizards compared to rodents do not have incisors that can be used to hold the animal.
(c) Lizard body holder. Three screws and a space for the head holder are located at the top of the structure. By sliding the head holder through the space, the position of the lizard can be adjusted.
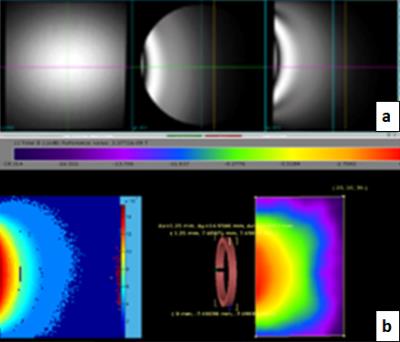
Figure 2: (a) Phantom image obtained for the B1 mapping. 1% Magnevist-distilled water solution was used for the scanning.
(b) Acquired B1 map being compared to the XFdtd simulation. The comparison shows that the result obtained from the B1 mapping reflects the expected B1 distribution obtained from the simulation.