4313
Measurement of Phosphocreatine and BOLD Kinetics in the Lower Extremity Muscles using a Dual-Frequency Coil Array1Center for Advanced Imaging Innovation and Research (CAI2R), New York University School of Medicine, New York, NY, United States, 2Bernard and Irene Schwartz Center for Biomedical Imaging, New York University School of Medicine, New York, NY, United States
Synopsis
MRI provides the unique ability to study metabolic and microvasculature functions in skeletal muscle using phosphorus and proton measurements. However, the low sensitivity of these techniques can make it difficult to capture dynamic muscle activity due to the temporal resolution required for kinetic measurements during and after exercise tasks. We developed a dual-nuclei coil array to enable proton and phosphorus MRI of the human lower extremities with SNR more than double that of a birdcage coil in the gastrocnemius muscles. This enabled the local assessment of phosphocreatine recovery kinetics following a plantar flexion exercise using an efficient sampling scheme with a 6 s temporal resolution. The integrated proton array demonstrated image quality approximately equal to that of a clinical state-of-the-art knee coil, which enabled fat quantification and dynamic blood oxygen level-dependent measurements that reflect microvasculature function.
Introduction
In vivo phosphorus magnetic resonance (31P-MR) has been used to assess phosphocreatine (PCr) and inorganic phosphate (Pi) kinetics that yield valuable information about muscle mitochondrial function during exercise1-4. Exercise protocols require suitable temporal resolution to capture mitochondrial function but can suffer from low SNR due to the low concentration of phosphorus-containing metabolites in human tissue5 and the low intrinsic sensitivity of 31P-MR. Proton MRI is also essential for assessing micro- and macro-vascular functions in skeletal muscle using dynamic techniques such as blood oxygenation level dependent (BOLD) MRI6 and arterial spin labeling (ASL)7. To enable both 31P and 1H-MR, we developed a dual-nuclei array8-14 for lower extremity MRI with encompassing eight-channel modules to provide volumetric coverage of the lower extremities along with a high SNR that can be exchanged for temporal resolution.Methods
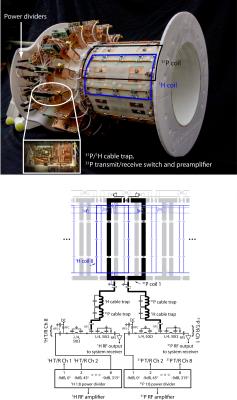
Two (31P and 1H) encircling transmit/receive arrays made up of eight loop coils each were constructed on a 17-cm-diameter housing structure. The 1H array was offset in the azimuthal direction by 22.5° (see Figure 1) to reduce shielding caused by the 31P array. All coils were tuned to 49.9 MHz (31P) or 123.2 MHz (1H) and matched to 50 Ω. The coils were loaded with a water-based gel phantom15 with dielectric properties that were designed to mimic muscle tissue16. Neighboring and next-nearest coils were decoupled via geometric overlap17 and lumped element inductors, respectively.
To generate circularly polarized B1+ fields, we drove the 31P and 1H arrays using separate eight-way power splitters that each consisted of three stages of Wilkinson power dividers and quadrature hybrids arranged to provide outputs with 45° phase offsets that corresponded to the azimuthal position of the coils. Individual power splitter outputs were connected to transmit/receive switches to protect the preamplifiers during transmission. Cable traps tuned the 31P and 1H frequencies were inserted between the transmit/receive switches and coil ports.
All imaging experiments were performed on a 3 Tesla MRI scanner (Prisma, Siemens). The study was approved by our IRB and human subjects were scanned after obtaining their informed written consent. We restricted transmit power to 10 W/kg based on MR thermometry measurements using a procedure similar to the method described in Ref. 18.
Results
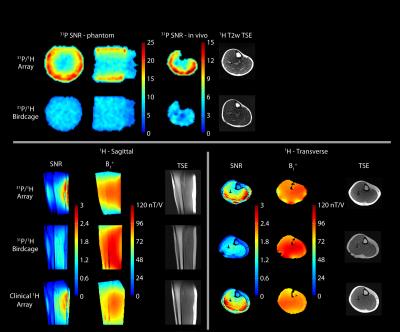
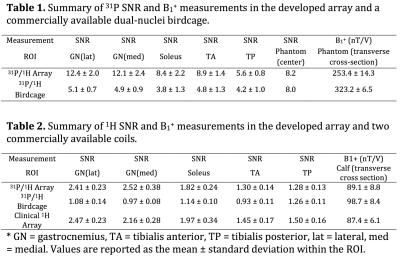
The in vivo SNR provided by the 31P module of the developed eight-channel array was more than 2.4 times greater than that of the commercially available birdcage coil in peripheral muscles and ~30% in the center (see Figure 2 and Tables 1 and 2). The 1H module provided a similar SNR advantage over the dual-nuclei birdcage coil and was within 15% of a state-of-the-art 15-channel mono-nuclear clinical array. The spin echo anatomical images exhibited good quality with no signs of artifacts.
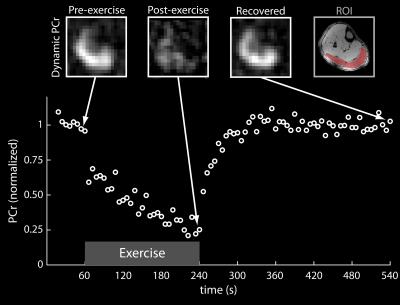
PCr recovery kinetics during an exercise protocol were acquired using a spectrally selective 31P-FLORET pulse sequence with a 6-s temporal resolution (Figure 3), which is comparable to the resolution obtained using unlocalized spectroscopy19-21.The PCr signal in the gastrocnemius muscle was fit to a single exponential recovery function to determine PCr depletion (77%) and the PCr resynthesis rate (kPCr = 20.5 s, r2 = 0.91).
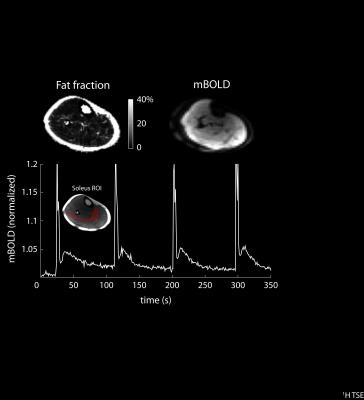
BOLD changes in the soleus muscle were measured during a 10 min period while the subject performed 1-s voluntary maximal isometric plantar flexions every 90 s. The spikes in the BOLD signal during the contractions were followed by delayed transient signal increases (see Figure 4). The relative BOLD signal increase (DSmax) was 2.9%, while the time-to-peak (TTP) was 12.5 s. Fat fraction maps calculated using the Hierarchical IDEAL method22 in a 31-year-old male volunteer with a BMI of 25.5 (see Figure 4) showed low fat infiltration (< 2.5%) in all muscle groups of the lower leg.
Discussion
The developed array provided a 31P SNR gain of 1.3- to 2.4-fold in vivo. We combined the coil with efficient FLORET sampling to measure PCr kinetics following exercise with a 6-s temporal resolution. In transmit mode, we drove the structure with circular polarization to generate an excitation field with a uniformity similar to that of the reference birdcage, which may eliminate the need for adiabatic excitation pulses that can be very lengthy, particularly when inverting deep lying spins. The array additionally provided 1H imaging and B0 shimming capabilities that allow several aspects of muscle function to be investigated, including regional perfusion,23 blood tissue oxygenation, and fat infiltration6,24.Acknowledgements
The authors thank Karthik Lakshmanan for insightful discussions on coil design, Guillaume Madelin for the FLORET pulse sequence, Xuejiao Che for assistance with transmit/receive switches, Riccardo Lattanzi for the SNR calculation script, and Jerzy Walczyk for construction of the coil housing. This work was partially supported by NIH grant R01DK106292 and was performed under the rubric of the Center for Advanced Imaging Innovation and Research (CAI2R, www.cai2r.net) at the New York University School of Medicine, which is an NIBIB Biomedical Technology Resource Center (NIH P41 EB017183). RB discloses the US patent, “Multi-Nuclei MRI Coil,” 13/866,728,2013, which is related to this work. PP and OK declare no financial conflicts of interest.
A full description of this work is available in: Brown, R. et al. Magnetic Resonance Imaging of Phosphocreatine and Determination of BOLD Kinetics in Lower Extremity Muscles using a Dual-Frequency Coil Array. Sci. Rep. 6, 30568; doi: 10.1038/srep30568 (2016).
References
1 Kemp, G., Ahmad, R., Nicolay, K. & Prompers, J. Quantification of skeletal muscle mitochondrial function by 31P magnetic resonance spectroscopy techniques: a quantitative review. Acta Physiologica 213, 107-144 (2015).
2 Arnold, D. L., Matthews, P. M. & Radda, G. K. Metabolic recovery after exercise and the assessment of mitochondrial function invivo in human skeletal-muscle by means of 31P NMR. Magnetic Resonance in Medicine 1, 307-315, doi:10.1002/mrm.1910010303 (1984).
3 Kemp, G. J., Taylor, D. J. & Radda, G. K. Control of phosphocreatine resynthesis during recovery from exercise in human skeletal-muscle. Nmr in Biomedicine 6, 66-72, doi:10.1002/nbm.1940060111 (1993).
4 Prompers, J. J. et al. Dynamic MRS and MRI of skeletal muscle function and biomechanics. NMR in Biomedicine 19, 927-953, doi:10.1002/nbm.1095 (2006).
5 Kemp, G. J., Meyerspeer, M. & Moser, E. Absolute quantification of phosphorus metabolite concentrations in human muscle in vivo by P-31 MRS: a quantitative review. Nmr in Biomedicine 20, 555-565, doi:10.1002/nbm.1192 (2007).
6 Towse, T. F. et al. Comparison of muscle BOLD responses to arterial occlusion at 3 and 7 Tesla. Magnetic Resonance in Medicine (2015).
7 Englund, E. K. et al. Multiparametric Assessment of Vascular Function in Peripheral Artery Disease Dynamic Measurement of Skeletal Muscle Perfusion, Blood-Oxygen-Level Dependent Signal, and Venous Oxygen Saturation. Circulation: Cardiovascular Imaging 8, e002673 (2015).
8 Goluch, S. et al. A form-fitted three channel 31P, two channel 1H transceiver coil array for calf muscle studies at 7 T. Magnetic Resonance in Medicine 73, 2376-2389 (2015).
9 Avdievich, N. I. & Hetherington, H. P. 4 T Actively detuneable double-tuned 1H/31P head volume coil and four-channel 31P phased array for human brain spectroscopy. J Magn Reson 186, 341-346, doi:10.1016/j.jmr.2007.03.001 (2007).
10 van der Velden, T. A. et al. Radiofrequency configuration to facilitate bilateral breast 31P MR spectroscopic imaging and high-resolution MRI at 7 Tesla. Magn Reson Med, doi:10.1002/mrm.25573 (2014).
11 Mirkes, C. et al. P CSI of the human brain in healthy subjects and tumor patients at 9.4 T with a three-layered multi-nuclear coil: initial results. Magma, doi:10.1007/s10334-016-0524-9 (2016).
12 Brown, R., Lakshmanan, K., Madelin, G. & Parasoglou, P. A nested phosphorus and proton coil array for brain magnetic resonance imaging and spectroscopy. Neuroimage 124, 602-611, doi:10.1016/j.neuroimage.2015.08.066 (2016).
13 Hardy, C. J., Bottomley, P. A., Rohling, K. W. & Roemer, P. B. An NMR phased array for human cardiac 31P spectroscopy. Magn Reson Med 28, 54-64 (1992).
14 Panda, A. et al. Phosphorus liver MRSI at 3 T using a novel dual-tuned eight-channel (3)(1)P/(1)H H coil. Magn Reson Med 68, 1346-1356, doi:10.1002/mrm.24164 (2012).
15 Duan, Q. et al. Characterization of a dielectric phantom for high-field magnetic resonance imaging applications. Med Phys 41, 102303, doi:10.1118/1.4895823 (2014).
16 Gabriel, S., Lau, R. W. & Gabriel, C. The dielectric properties of biological tissues: III. Parametric models for the dielectric spectrum of tissues. Phys Med Biol 41, 2271-2293 (1996).
17 Roemer, P. B., Edelstein, W. A., Hayes, C. E., Souza, S. P. & Mueller, O. M. The NMR phased array. Magn. Reson. Med. 16, 192-225 (1990).
18 Brown, R. et al. Design and application of combined 8-channel transmit and 10-channel receive arrays and radiofrequency shimming for 7-T shoulder magnetic resonance imaging. Invest Radiol 49, 35-47, doi:10.1097/RLI.0b013e3182a5662d (2014).
19 Meyerspeer, M. et al. Simultaneous and interleaved acquisition of NMR signals from different nuclei with a clinical MRI scanner. Magnetic resonance in medicine (2015).
20 Parasoglou, P., Feng, L., Xia, D., Otazo, R. & Regatte, R. R. Rapid 3D-Imaging of Phosphocreatine Recovery Kinetics in the Human Lower Leg Muscles with Compressed Sensing. Magnetic Resonance in Medicine 68, 1738–1746 (2012).
21 Parasoglou, P., Xia, D., Chang, G. & Regatte, R. R. Dynamic three-dimensional imaging of phosphocreatine recovery kinetics in the human lower leg muscles at 3T and 7T: a preliminary study. NMR Biomed 26, 348-356, doi:10.1002/nbm.2866 (2013).
22 Tsao, J. & Jiang, Y. Hierarchical IDEAL: Fast, robust, and multiresolution separation of multiple chemical species from multiple echo times. Magnetic Resonance in Medicine 70, 155-150, doi:10.1002/mrm.24441 (2012).
23 Englund, E. K. et al. Combined measurement of perfusion, venous oxygen saturation, and skeletal muscle T2* during reactive hyperemia in the leg. Journal of Cardiovascular Magnetic Resonance 15, 70 (2013).
24 Slade, J. M., Towse, T. F., Gossain, V. V. & Meyer, R. A. Peripheral microvascular response to muscle contraction is unaltered by early diabetes but decreases with age. J Appl Physiol 111, 1361-1371 (2011).
25 Gold, G. E. et al. Musculoskeletal MRI at 3.0 T: relaxation times and image contrast. AJR Am J Roentgenol 183, 343-351, doi:10.2214/ajr.183.2.1830343 (2004).
Figures