4307
Occipital-parietal coil with variable-density element distribution for 7T functional imaging1Centre for Functional and Metabolic Mapping, The University of Western Ontario, London, ON, Canada
Synopsis
A 32-channel receive coil with variable-density element distribution, in conjunction with an 8-channel transmit coil, was developed for imaging of the occipital-parietal regions of human brain at 7T. Spatial SNR maps demonstrate targeted sensitivity to the peripheral occipital pole, with a smoothly varying SNR proximal to this region. The temporal SNR of the occipital-parietal coil attained 35% higher SNR in the visual cortex than a whole-head coil.
Purpose
Functional imaging during visual experiments requires high SNR in the visual cortex, while concurrently attaining a sufficient field-of-view (FOV) for the effective implementation of motion correction and registration algorithms. Coils that limit the FOV to the occipital and parietal lobes as a means to increase SNR in visual experiments have been implemented at 3T1 and 7T2. This study expands upon these designs by adapting the size, and therefore density, of receive elements based upon their proximity to the primary visual cortex, in order to manipulate the SNR spatial profile. A larger 8-channel transmit coil is used to increase the flip-angle uniformity within the region of interest.Methods
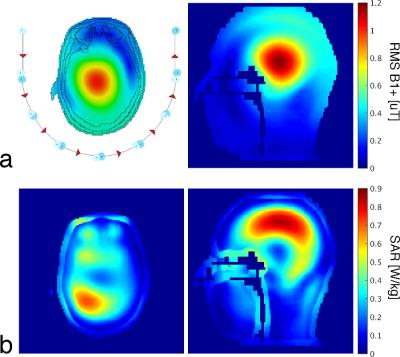
The transmit coil consisted of eight independently driven overlapped rectangular loops (8.7 cm by 22.5 cm). Transmit loops extended further in the anterior direction than the receive coil; this reduced asymmetry in the flip-angle distribution, over the desired imaging volume, caused by the asymmetric transmit field profile of individual loops at high field strengths. To evaluate SAR, full-wave electromagnetic simulations of the transmit coil were performed when loaded with a human head model.
The receive coil was comprised of 32 loops ranging in diameter from 52 mm to 104 mm (Fig. 1). The smallest loops were located over the primary visual cortex to create the highest peripheral SNR in this location. Loops were arranged in approximately concentric rings about the primary visual cortex, with each ring having loops of larger diameter; this increased the penetration depth for these elements and created a larger useable imaging region. The receive coil measured 19.5 cm (left-right), 16.5 cm (anterior-posterior), and 22.7 cm (head-foot). Elements were tuned to 297.2 MHz and connected to matching boards via lambda/2 electrical lengths, which included a coaxial cable and a phase shifter. Matching boards had active and passing detuning networks, a serial capacitor for preamplifier decoupling, a fast-blow fuse, and a matching capacitor. Low-input-impedance preamplifiers were mounted to the matching boards. A large mirror mounted above the eyes, in conjunction with a projection screen mounted directly on the back of the coil, resulted in an unobstructed 35° - 40° visual angle for the subject.
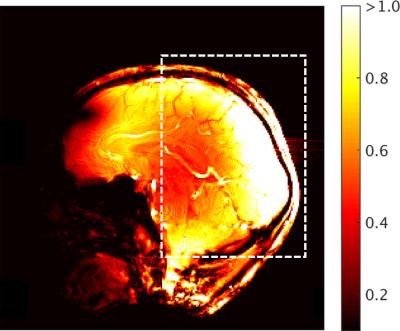
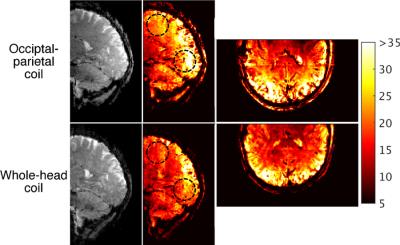
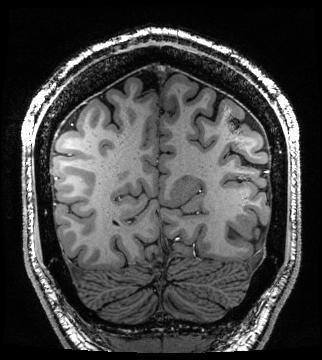
The spatial distribution of SNR was measured in the sagittal plane to evaluate the effect of the variable loop density. Spatial SNR was derived from non-accelerated gradient-echo acquisitions, with and without RF transmission. Temporal SNR was compared to an 8-channel transmit/32-channel receive coil3 that had whole-head coverage (resolution: 1.5-mm isotropic, number of slices: 75, TE/TR: 20/1236 ms, flip angle: 45°, bandwidth: 1576 Hz/pixel). EPI time series were accelerated with an iPat of 3 (with 36 reference lines) and a multiband4 factor of 3. Image quality was assessed with an MPRAGE image acquired with 500-μm isotropic resolution (TE/TR/TI: 3.6/3000/1200 ms, flip: 9°, bandwidth: 130 Hz/pixel).
Results and Discussion
The transmit coil had an average S12 of -17 dB between adjacent coil elements. Figure 2 shows representative axial and sagittal slices of a simulated B1+ efficiency map and the corresponding 10-g-averaged local SAR map. It is notable that the highest local SAR does not occur in the eyes, which is common with transmit coils that extend around the entire head.
Preamplifier decoupling and geometric decoupling resulted in a mean and maximum noise correlation of 9.5% and 42%, respectively, when acquired with a 50-kHz bandwidth. The smallest receive element, located over the primary visual cortex, had a Q-ratio of 4.5.
The occipital-parietal coil produced its highest SNR in the primary visual cortex (Fig. 3). As per the design expectations, the SNR smoothly decreased as a function of distance from this focal point. The occipital-parietal coil attained a higher temporal SNR (Fig. 4) than the whole-head coil in both the occipital lobe (35%) and parietal lobe (21%). As expected, the advantage of the whole-head begins to decrease toward the anterior aspect of the head. A coronal slice of an MPRAGE image shows high SNR with uniform contrast between grey and white matter (Fig. 5).
Conclusions
The variable density of receive elements produced the maximum SNR in the peripheral visual cortex with smoothly varying SNR in the parietal lobe and cerebellum. The receive coil layout, when combined with a multi-channel coil for transmission, is ideal for functional experiments targeting the visual system at ultra-high field strengths.Acknowledgements
No acknowledgement found.References
1. Farivar R, Grigorov F, van der Kouwe AJ, Wald LL, Keil B. Dense, shape-optimized posterior 32-channel coil for submillimeter functional imaging of visual cortex at 3T. Magn Reson Med 2016; 76: 321-328.
2. Adriany G, Waks M, Tramm B, Schillak S, Yacoub E, de Martino F, van de Moortele P-F, Naselaris T, Olman C, Vaughan T, Ugurbil K. An open faced 4 ch. Loop transmit / 16 ch. Receive array coil for hires fMRI at 7 Tesla. Proceedings of the 20th Annual Meeting of ISMRM, Melbourne, Australia, 2012; 429.
3. Gilbert KM, Gati JS, Kho E, Klassen LM, Zeeman P, Menon RS. An parallel-transmit, parallel-receive coil for routine scanning on a 7T head-only scanner. Proceedings of the 23rd Annual Meeting ISMRM, Toronto, Canada, 2015; 623.
4. Moeller S, Yacoub E, Olman CA, Auerbach E, Strupp J, Harel N, Ugurbil K. Multiband multislice GE-EPI at 7 Tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain FMRI. Magn Reson Med 2010; 63(5): 1144-1153.
Figures