4305
A 8-channel pTx transceive coil for hip imaging at 7 T1University of Queensland, St Lucia, Australia, 2Siemens Ltd, Brisbane, Australia, Australia, 3American University of the Middle East, Kuwait
Synopsis
This paper presents the initial in vivo imaging results from a new unilateral 8-channel pTx transceive array designed and constructed for 7 T MRI of the hip joint. With subject-specific RF shims, 3D sequences (DESS and MEDIC) provided sufficient coverage, uniform image intensity and excellent contrast. The RF techniques employed in the construction of the array promote efficient use of RF power, enabling turbo spin echo sequences with full coverage of hip joint to be performed with adequate excitation. Comprehensive and conservative RF safety procedures ensure that local RF energy absorption is well below regulatory limits.
PURPOSE:
In musculoskeletal MRI, ultrahigh-field (UHF) systems offer superior SNR, enabling higher spatial resolution for improved assessment of joints and associated tissues (e.g., menisci, ligaments and cartilage). At UHF, however, adequate flip-angle (FA) excitations are limited by the available RF power from of a standard system (typically 8 kW) and by the specific absorption rate (SAR) limits. Additionally, inhomogeneous FA distributions are more pronounced at UHF, which can significantly degrade image quality. These two effects become more severe when imaging large body regions and/or deeply seated anatomies (e.g., hip joint). The use of RF parallel transmission (pTx) techniques provides a promising solution as per recent pTx coil developments 1-7. This work presents the initial in vivo imaging results from a new 8-channel pTx transceive array designed and constructed for unilateral imaging of the hip joint at 7 T.METHODS:
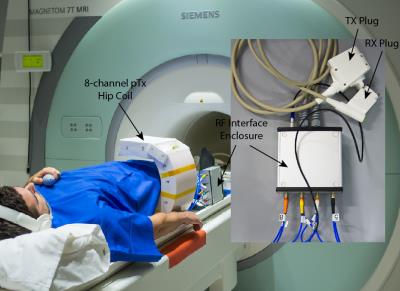
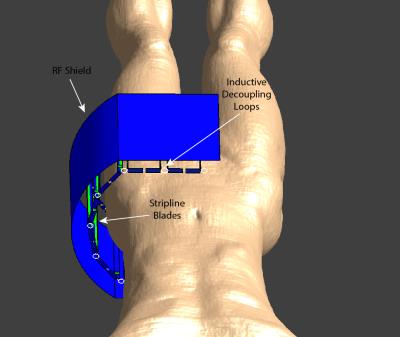
The constructed hip pTx coil is an 8-loop array arranged in a C-shape of single cylindrical row with an opening of 280 mm (Fig.1). An RF shield was placed inside the coil housing 30 mm radially away from the elements. The elements (length: 180 mm, width: 68 mm) were made of multi-layered stripline-like conductors 8 to promote uniform distribution of currents and efficient transmission. They are angularly oriented 9 to optimise transmit/receive profiles. Counter-wound inductive loops 10 were used to decouple the coil elements, achieving coil-coil decoupling better than -18 dB (port matching to 50 Ω better than -20 dB) for a typical load as measured with a network analyser. The coil-array was interfaced to the MRI system through a dedicated 8-channel Tx/Rx switch 11.
Electromagnetic simulations with two anatomically accurate voxel models (Duke and Ella 12) were performed using Sim4Life by ZMT 13 (Fig.2). The coil elements and voxel models were meshed with irregular grids to best represent geometries. Lumped elements were used to fine tune the resonance frequency and to match the input impedance to 50 Ω (reflection coefficient S11 better than -20 dB for all channels). Perfect decoupling was assumed by simulating each channel independently with a converge to -40 dB. Individual electric field distributions of 1 V harmonic sources were used for calculating the virtual observation points (VOP) 14 for in vivo worst-case local SAR monitoring.
Two healthy volunteers were imaged with the pTx hip coil on a 7 T whole-body research scanner (Siemens Healthcare, Erlangen, Germany) with informed written consent. Imaging sequences included a gradient echo (GRE) field map for B0 shimming, a turbo spin echo (TSE) with proton density-weighting (PDw), PDw with fat-saturation (PDw-FatSat), T1-weighting (T1w) and T2-weighting (T2w), 3D water-excited Dual-Echo Steady-State (we-DESS) and 3D Multi-Echo Data Image Combination (MEDIC). Optimal B1 shims were calculated on a subject-specific basis using the vendor-supplied toolbox (Syngo VB17) and acquired B1 maps. The shimming volume included the head, neck, and proximal shaft of the femur.
RESULTS:
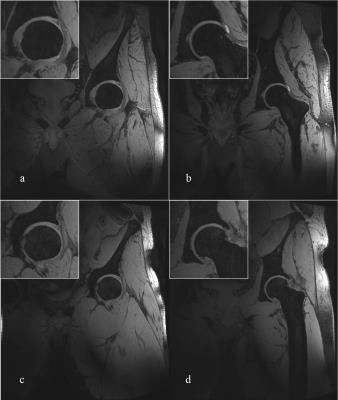
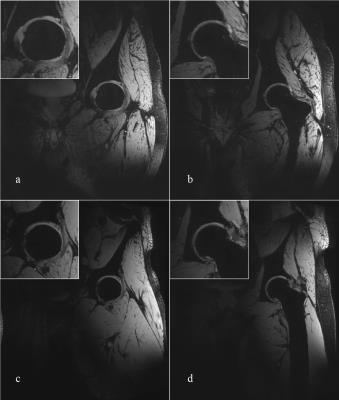
Representative coronal slices of the hip from 3D we-DESS and MEDIC images are shown in Figs.3 and 4, respectively. Both images were taken with 0.56 mm isotropic resolution and a field of view (FOV) of 320 mm with TE/TR of 3.1/11 and 13/20 ms for DESS and MEDIC, respectively (respective TA = 4:01 and 6:26 min).
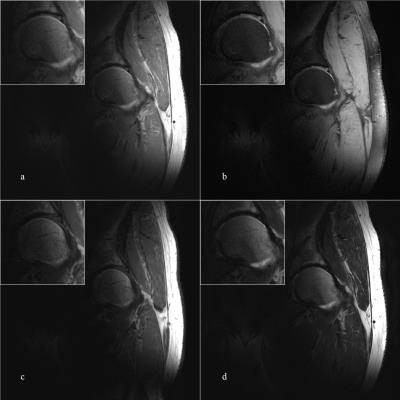
Fig.5 illustrates a representative coronal TSE images (25 slices) of the hip joints (images of the second volunteer not shown). The TSE images had a FOV of 256 mm and an in-plane resolution of 0.3 × 0.3 mm2 (slice thickness = 3 mm). TE/TR were adjusted to achieve PDw (24/5200 ms, Figs.5a and 5b), T1w (12/900 ms, Fig.5c), and T2w (86/9000 ms, Fig.5d). Parallel imaging (reduction factor R = 2) and varied turbo factors were employed for TAs of the TSE sequences to be below 4 mins. The highest worst-case local-SAR estimation occurred in the T1w sequence at 9.91 W/kg.
DISCUSSION:
High-quality 2D (TSE) and 3D (DESS, MEDIC) images of the hip were acquired from the new pTx array. The 3D sequences provided good visualisation of the joint cartilage and surrounding fluids, whilst the cortical bone showed a hypointensive signal. The separation of the individual articular cartilage plates was better defined in the MEDIC images. Combined with successful individualized shims, the coil array provided excellent penetration (2/3 of the body width), and relatively homogenous image intensity over the region of interest for the 3D sequences. In comparison, TSE sequences were more sensitive to B1 inhomogeneity, which can be addressed in the future with better slice-specific B1 shims or dedicated RF pulses.CONCLUSION:
Initial high quality images were presented for a new unilateral hip pTx array.Acknowledgements
We acknowledge ZMT Zurich MedTech AG (www.zurichmedtech.com) for providing the electromagnetic simulation software package SIM4LIFE.References
1. C.J. Snyder, L. DelaBarre, S. Moeller, et al., Comparison between eight- and sixteen-channel TEM transceive arrays for body imaging at 7 T. Magnetic Resonance in Medicine, 2012. 67(4): p. 954-964.
2. J.M. Theysohn, O. Kraff, S. Orzada, et al., Bilateral hip imaging at 7 Tesla using a multi-channel transmit technology: initial results presenting anatomical detail in healthy volunteers and pathological changes in patients with avascular necrosis of the femoral head. Skeletal Radiology, 2013. 42(11): p. 1555-1563.
3. A. Graessl, W. Renz, F. Hezel, et al., Modular 32-channel transceiver coil array for cardiac MRI at 7.0T. Magnetic Resonance in Medicine, 2014. 72(1): p. 276-290.
4. Ö. Ipek, A.J. Raaijmakers, J.J. Lagendijk, et al., Intersubject local SAR variation for 7T prostate MR imaging with an eight-channel single-side adapted dipole antenna array. Magnetic Resonance in Medicine, 2014. 71(4): p. 1559-1567.
5. J.M. Theysohn, O. Kraff, N. Theysohn, et al., Hip imaging of avascular necrosis at 7 Tesla compared with 3 Tesla. Skeletal Radiology, 2014. 43(5): p. 623-632.
6. O. Kraff, A. Fischer, A.M. Nagel, et al., MRI at 7 tesla and above: Demonstrated and potential capabilities. Journal of Magnetic Resonance Imaging, 2015. 41(1): p. 13-33.
7. C. Oezerdem, L. Winter, A. Graessl, et al., 16-channel bow tie antenna transceiver array for cardiac MR at 7.0 tesla. Magnetic Resonance in Medicine, 2016. 75(6): p. 2553-2565.
8. E. Weber, B.K. Li, F. Liu, et al., "A ultra high field multi-element transceive volume array for small animal MRI," in Engineering in Medicine and Biology Society, 2008. EMBS 2008. 30th Annual International Conference of the IEEE, 2008, pp. 2039-2042.
9. B.K. Li, F. Liu, E. Weber, et al., Hybrid numerical techniques for the modelling of radiofrequency coils in MRI. NMR in Biomedicine, 2009. 22(9): p. 937-951.
10. S. Crozier, B.K. Li, F. Liu, et al., MRI coil design. 2009, Google Patents.
11. E. Weber, C. Engstrom, K. O'Brien, et al., "An open 8-channel pTx coil for 7-Tesla MRI of the knee and ankle joints at multiple postures," in Proceedings of the 25th Annual Meeting of ISMRM, Honolulu, HI, USA, 2017, p. 2936.
12. C. Andreas, K. Wolfgang, G.H. Eckhart, et al., The Virtual Family—development of surface-based anatomical models of two adults and two children for dosimetric simulations. Physics in Medicine and Biology, 2010. 55(2): p. N23.
13. SIM4LIFE by ZMT. Available: www.zurichmedtech.com
14. G. Eichfelder and M. Gebhardt, Local specific absorption rate control for parallel transmission by virtual observation points. Magnetic Resonance in Medicine, 2011. 66(5): p. 1468-1476.
Figures