4278
Both Rostral and Dorsal Anterior Cingulate Cortices Exhibit Age-related Metabolic Changes1Department of Diagnostic Radiology, The University of Hong Kong, Hong Kong, Hong Kong, 2State Key Laboratory of Brain and Cognitive Sciences, The University of Hong Kong, Hong Kong, Hong Kong, 3Department of Special Education and Counselling, The Education University of Hong Kong, Hong Kong, Hong Kong, 4Sau Po Centre on Ageing, The University of Hong Kong, Hong Kong, Hong Kong, 5Department of Social Work and Administration, The University of Hong Kong, Hong Kong, Hong Kong, 6Philips Healthcare, Hong Kong, Hong Kong, Hong Kong, 7Alzheimer's Disease Research Network, The University of Hong Kong, Hong Kong, Hong Kong
Synopsis
Rostral ACC displays a characteristic task-induced deactivation, while dorsal ACC displays positive BOLD responses in cognitive tasks. Nevertheless, the effect of age on the rostral ACC and dorsal ACC has never been investigated within the same cohort. In this study, quantitative proton magnetic resonance spectroscopy was used to investigate the metabolic changes in the rostral ACC and dorsal ACC in a local Chinese cohort at 3.0T. Both rostral ACC and dorsal ACC showed age-related metabolic changes. Rostral ACC may reveal greater degree of compensation compared to dorsal ACC.
Purpose
The anterior cingulate cortex (ACC) has recently become a focus for aging research because of its implicated role in cognition, and its involvement in the default mode network (DMN), which is a set of brain regions that are active during resting state and such activity were reported to be decreased in healthy elderly adults compared to younger adults.1 It is notable that the ACC can be divided into rostral (perigenual) ACC, which belongs to part of the DMN and displays a characteristic task-induced deactivation,2,3 and dorsal (supragenual) ACC, which displays positive blood oxygen level-dependent fMRI responses in cognitive tasks.4,5 Despite their differential role, the effect of age on the rostral ACC and dorsal ACC has never been studied. In this study, we investigated the metabolic changes during aging in the rostral ACC and dorsal ACC of a local Chinese cohort using quantitative proton magnetic resonance spectroscopy (1H-MRS). Regional differences between rostral ACC and dorsal were also assessed.Methods
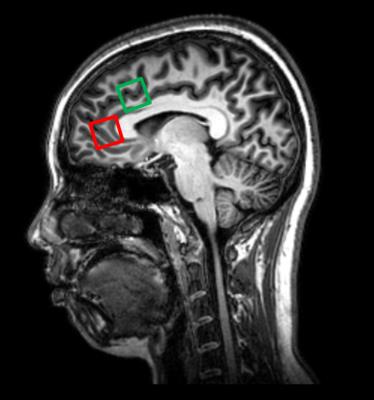
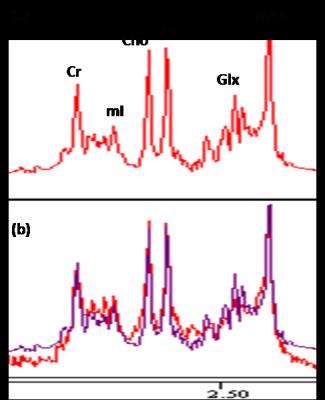
36 cognitively normal (Mini-mental State Examination≥28; Montreal Cognitive Assessment≥26) subjects (mean = 49.3±17.5 years, age range 24-84 years) underwent MR scan using 3.0T Philips scanner. Two PRESS (TR/TE = 2000/39 ms) single voxels of 2 x 2 x 2 cm3 were placed in the rostral ACC and dorsal ACC (Figure 1). Choline (Cho), creatine (Cr), N-acetyl aspartate (NAA), myo-inositol (mI), and summation of glutamate and glutamine complex (Glx), were measured and quantified using internal water as reference by QUEST in jMRUI (4.0) (Figure 2). Cerebrospinal fluid (CSF) normalization, water content correction for grey matter, white matter and CSF, and correction factors for T1 and T2 relaxation were also implemented. Pearson correlation coefficient (r) was calculated to assess any correlation between absolute metabolite concentrations and age in both rostral ACC and dorsal ACC. Paired t-test was used to investigate any regional differences in rostral ACC and dorsal ACC. SPSS version 20.0 was used for statistical analysis and level of significance was set at 0.05.Results
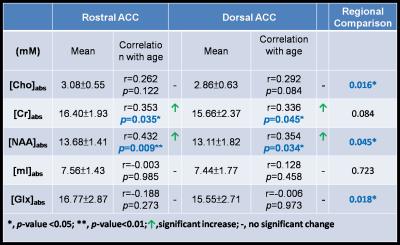
Table 1 shows mean absolute metabolite concentrations, correlation with age in both rostral ACC and dorsal ACC, and the regional metabolite concentrations comparison. In rostral ACC, absolute concentrations of Cr ([Cr]abs) (r = 0.353; p = 0.035) and [NAA]abs (r = 0.432; p = 0.009) showed significant positive correlations with age, whereas in dorsal ACC, [Cr]abs (r = 0.336; p = 0.045) and [NAA]abs (r = 0.354; p = 0.034) also showed significant positive correlation with age. Paired t-test showed that rostral ACC possessed significantly higher amount of [Cho]abs (p=0.016), [NAA]abs (p=0.045), and [Glx]abs (p=0.018), compared to dorsal ACC.Discussion
Age-related changes of [Cr]abs and [NAA]abs in both rostral and dorsal ACC
Our findings of age-related increases of [Cr]abs and [NAA]abs in both rostral ACC and dorsal ACC are consistent with prior studies.6,7 Age-related increase in [Cr]abs might imply elevating number of glial cells, while increase in [NAA]abs with age might indicate compensation activity. Our finding might then shed light on providing an explanation on the fMRI changes in aging as a prior fMRI study involving the colour Stroop task showed an increased activation of the ACC in older participants compared to young adults.8
Higher [Cho]abs, [NAA]abs, and [Glx]abs in rostral ACC
Our findings of significantly higher [Cho]abs, [NAA]abs, and [Glx]abs shown in rostral ACC compared to dorsal ACC might high-light the importance of rostral ACC in the DMN since the ACC is also connected to the fronto-insular cortex (FIC), forming the FIC-ACC network, together possessing a specialized class of neurons to facilitate switching process between the DMN and cognitively demanding tasks.9
Conclusion
Both rostral ACC and dorsal ACC showed increased [Cr]abs and [NAA]abs with age suggesting compensation. Rostral ACC may reveal greater degree of compensation compared to dorsal ACC.Acknowledgements
No acknowledgement found.References
1. Damoiseaux JS, Beckmann CF,Sanz Arigita EJ, et al. Reduced resting-state brain activity in the “default network” in normal aging. Cerebral Cortex 2008;18 (8):1856-1864
2. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann NY Acad Sci 2008;1124:1–38.
3. Northoff G, Walter M, Schulte RF, et al. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat Neurosci 2007;10:1515–1517.
4. Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: An update of theory and data. Cogn Affect Behav Neurosci 2007;7:367–379.
5. Duncan NW, Enzi B, Wiebking C, et al. Involvement of Glutamate in Rest-Stimulus Interaction Between Perigenual and Supragenual Anterior Cingulate Cortex: A Combined fMRI-MRS Study Human Brain Mapping 2011;32(12):2172-82
6. Charlton RA, McIntyre DJO, Howe FA, et al. The relationship between white matter brain metabolites and cognition in normal aging: the GENIE study. Brain Research 2007;1164:108–16
7. Schuff N, Ezekiel F, Gamst AC, et al. Region and tissue differences of metabolites in normally aged brain using multislice 1H magnetic resonance spectroscopic imaging. Magn Reson Med 2001;45:899–907
8. Milham MP, Erickson KI, Banich MT. et al. Attention control in the aging brain: insights from an fMRI study of the Stroop task. Brain Cogn 2002;49:277–96
9. Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. PNAS 2008;105 (35); 12569-12574
Figures