4276
Sub-clinical trait anxiety relates to cerebral blood flow in brain regions related to autonomic arousal1Radiological Sciences, Division of Clinical Neuroscience, University of Nottingham, Nottingham, United Kingdom, 2Arthritis Research UK Pain Centre, University of Nottingham, Nottingham, United Kingdom, 3Sir Peter Mansfield Imaging Centre, University of Nottingham, Nottingham, United Kingdom
Synopsis
Arterial spin labelling is a powerful, non-invasive tool to map cerebral blood flow (CBF) in the study of neural activity patterns underpinning spontaneous behaviour or personality traits. In this study we sought to directly investigate the effects of negative affect, specifically trait anxiety on local cerebral blood flow in a group of patients with chronic pain known to have mildly elevated anxiety scores. This study found widespread cerebral blood flow in osteoarthritis subjects with chronic pain that correlate significantly with trait anxiety, overlapping with regions previously reported to relate to autonomic functions. fMRI studies should account for increased physiological arousal.
Introduction
Arterial spin labelling is a powerful, non-invasive tool to map cerebral blood flow (CBF) in the study of neural activity patterns underpinning spontaneous behaviour or personality traits1,2. There is growing interest to use CBF mapping to investigate clinical symptoms that cannot be experimentally mimicked such as chronic pain3. Differences between the brain activation pattern induced by experimental pain and underpinning spontaneous/ongoing pain have been consistently reported4,5. Due to lack of control condition, the sensitive correlational analysis approach does however lack specificity and several factors associated with global or local CBF need to be considered when interpreting ASL-behavioural associations. We recently showed that limbic CBF correlations with intensity of chronic pain intensities became null when controlling for trait anxiety, and was partly reduced by controlling for depression scores3. In this study we sought to directly investigate the effects of negative affect, specifically trait anxiety on local cerebral blood flow in a group of patients with chronic pain known to have mildly elevated anxiety scores. We hypothesised that patients with higher anxiety levels tend to be more aroused during the MRI scanning leading to higher CBF in the sympathetic brain networks.Methods
89 individuals with chronic knee osteoarthritis pain (M ± SD: 65.8 ± 9.8, 46 males, VAS (0-100) 28.3 ± 22.2, average pain duration 5.3yrs) but no other co-morbidities were included in this study. All participants underwent a battery of questionnaires looking at pain and negative affect (including depression and anxiety). All patients underwent multimodal MRI at 3T (Discovery MR750, GE Healthcare) including a pulsed-continuous ASL (pCASL) with a 3D spiral read-out (Flip angle = 111°, TE/TR = 10.5/4632ms, labelling duration = 1525ms, FOV= 240mm, slice thickness = 4mm, slice gap = 4mm, number of slices = 36, echo train length = 1, excitations = 3 and matrix = 128 x 128). Cerebral blood flow maps (CBF) were generated using automatic reconstruction script6. CBF and T1-weighted images were brain extracted using FSL-BET v2.1 and then a two-step linear registration was performed CBF->T1 (6DOF), T1->MNI152 (12DOF) using FSL-FLIRT v6.0. The resulting images were then smoothed with an 8mm FWHM kernel using SPM12. As this study focusses upon grey matter CBF, we used a dual-tissue probability mask (as described previously3). To address the main study aim, trait anxiety scores were correlated with CBF images on a voxel-base. These correlations were corrected for whole-brain grey matter CBF to correct for inter-subject CBF differences of no interest using a GLM approach. Voxel-wise permutation testing was performed using FSL-randomise (5000 permutations) with significance defined as P<0.05, FWE-corrected using threshold-free cluster enhancement.Results
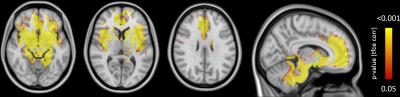
Correlating trait anxiety resulted in widespread significant clusters predominantly in regions overlapping with the salience network (mid-rostral anterior cingulate, bilateral insulae) as well as bilateral S2, basal ganglia, amygdala and thalami (Figure 1). All results passed significance (P<0.05 FWE).Discussion
This study has found widespread cerebral blood flow in osteoarthritis subjects with chronic pain that correlate significantly with trait anxiety, a measure of one’s predisposal to worry, stress, and anxiousness. As we hypothesised, the regions reported here overlap with those previously found to relate to autonomic functions7. For instance, a previous meta-analysis reported that the mid-cingulate, anterior and posterior insula and the amygdala were all consistently related to autonomic function in both parasympathetic and sympathetic arousal whilst we report them as significantly correlated with trait anxiety. In an elderly, diseased cohort such as the one investigated here, a confound such as increased anxiety (and its related increase in sympathetic arousal) is important to be taken into account due to the effect this could have on functional MRI outcomes. Increased anxiety (in comparison to healthy age-matched subjects) in chronic pain is commonly reported and in light of these results, should be better managed and measured in future studies (e.g. habituating to scanning environment using a mock scanner). As the anxiety scores reported here correspond to sub-clinical levels of anxiety, these results could also impact on fMRI studies more widely, highlighting the important effect that sympathetic arousal may have on results.Conclusion
In conclusion, this study displays how trait anxiety relates to CBF in brain regions underlying the salience network and related to autonomic processes. As our study cohort only reported mild (subclinical) anxiety this increase in autonomic arousal needs to be seriously considered and accounted for in both ASL and BOLD activation studies across healthy and patient studies so as to avoid unaccounted confounds.Acknowledgements
We would like to thank Arthritis Research UK for the financial support of this study and we are grateful to Nadia Frowd and Sharon Forman for their time and dedication in the running of the study.References
1. Wang J, et al (2005). PNAS. 102(49):17804-17809
2. O’Gorman RL, et al (2006). NeuroImage. 31: 489-495
3. Cottam WJ, et al (2016). NeuroImage:Clinical. 12: 269-276
4. Kulkarni B, et al (2007). Arthritis & Rheumatism. 56(4): 1345-1354
5. Parks EL, et al (2011). European Journal Of Pain. 15(8): 843:e1-14
6. Zaharchuk B, et al (2010). Magnetic Resonance in Medicine. 63: 1548-1556
7. Beissner F, et al (2013). The Journal Of Neuroscience. 33(25): 10503-10511