4274
Quantification of Brain Metabolites in Alcohol Dependent Patients Using MRS with Experimental Basis Sets at 3T1Korea Basic Sicence Institute, Ochang, Korea, Republic of, 2Dept. Bio-Analytical Science, Univ of Science & Technology, Daejeon, Korea, Republic of
Synopsis
In this study, in vivo quantification of brain metabolites measured on the dorsolateral prefrontal cortex (DLPFC) was carried out with LCModel using a priori knowledge based on experiment metabolite basis set signals. The main observation in this work was the significant reduction of tCho and Ins, and increase of GSH and Glx concentrations in the left DLPFC of alcohol dependent patients compared to healthy control subjects.
Purpose
In vivo proton magnetic resonance spectroscopy (1H-MRS) has been used as a tool to study not only metabolic disorders in patients, but also metabolic changes in healthy people1. Chronic alcoholism is associated with cognitive impairments affecting executive functions, verbal/visual memory, and visuospatial functions2. These impairments are moderate to severe but usually remain undiagnosed. In some of the previous studies. 1H-MRS has shown that alcoholic dependent subjects had lower levels of N-acetyl aspartate (NAA), phosphocreatine plus creatine (PCr+Cr, tCr), choline containing compounds (GPC+PC, tCho), and higher levels of glutamate plus glutamine (Glu+Gln, Glx) in the dorsolateral prefrontal cortex (DLPFC)3, 4. However, more studies are still needed to confirm those observations. In the present study, instead of measuring the ratio of metabolites, in vivo quantification of brain metabolites measured on the DLPFC was carried out with LCModel using a priori knowledge based on experiment metabolite basis set signals. Metabolic concentrations of alcohol-dependent patients were calculated and compared the findings with those for healthy controls.Methods
This study included 50 subjects, 26 male alcohol dependents (mean±SD, 51±8.3 years) and 24 healthy control subjects (mean±SD, 52±8.4 years). Single-voxel 1H-MRS was performed using a PRESS sequence at 3T (e.g., Philips Achieva TX System with a 32-channel receive-only array head coil). The examinations (voxel size, 2×2×2 cm3) were measured from left DLPFC in patients with alcohol dependents and healthy subjects. After shimming procedure, water suppression was accomplished with VAPOR(variable pulse power and optimized relaxation delays) pulses. The acquisition parameters were TR/TE = 2500/35 ms, and 128 acquisitions for averaging. A fully relaxed, unsuppressed spectrum was also acquired to measure the water peak (16 averages). LCModel (Linear combination of model spectra of metabolite solutions in vitro) fitting was conducted using in-house measured basis spectra of 16 metabolites. To evaluate the performance of experimental basis sets, Cramer-Rao lower bounds (CRLB) were evaluated. For more accurate metabolite quantification, cerebrospinal fluid (CSF) correction was performed. For estimation of CSF percentage in the VOI for each subject, statistical parametric mapping (SPM) was used for segmentation.Results
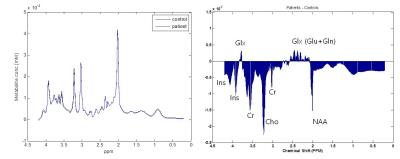
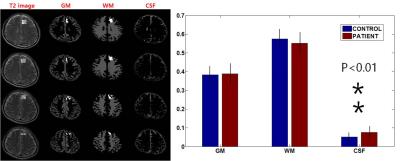
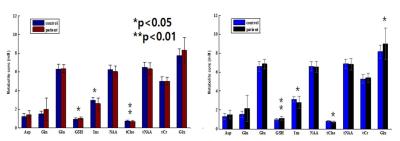
Figure 1 shows the averaged spectrum (Fig. 1 Left) of alcohol-dependent patients (blue color) and healthy controls (black color), and spectral difference between the two groups (Patients – Controls). In this study, a total of 10 metabolites could be quantified reliably (CRLB < 30%): NAA, N-acetylaspartate+N-acetylaspartylglutamate (tNAA), tCr, tCho, Glx, myo-inositol (Ins), glutathione (GSH), and aspartate (Asp). The estimated ratio of CSF in the region of interest was calculated to be about 5% (Figure 2) and after correction, concentration of each metabolite was increased. tCho concentration was significantly lower in DLPFC in alcohol-dependent patients (p < 0.05) than in the control subjects, which is consistent with the finding of previous study. However, there was no significant difference in the NAA concentration between the two groups (p = 0.31). In addition, alcoholic dependent patients had lower level of Ins, and higher levels of Glx and GSH in the dorsolateral prefrontal cortex in this study (p < 0.05, p < 0.05, and p < 0.01 in Figure 3).Discussion/Conclusion
The present study demonstrated that in vivo 1H-MRS can be used to detect the brain metabolite abnormalities in alcoholic dependents. The main observation in this work was the significant reduction of tCho and Ins, and increase of GSH and Glx concentrations in the left DLPFC of alcohol dependent patients compared to healthy control subjects. tCho levels are lower in prefrontal brain areas of recently detoxified alcoholic patients4. In this study, the decreased tCho levels are interpreted as indicating damage to cell membranes and/or myelin caused by chronic alcohol consumption. The function of Ins is not well understood, but it is associated with cell growth, osmolite, and a storage form for glucose5. The decreased Ins levels might reflect functional changes with a reduction of glucose metabolism or cerebral blood flow in prefrontal regions in alcohol dependent patients. GSH, a tripeptide composed of glycine, cysteine, and Glu, serves important cofactor roles in antioxidant defense and drug detoxification. It may serve as a reservoir of neural Glu in an amino acid transport system. Thus, increased Glu level followed inhibition of GSH synthesis temporally may precede later effects upon oxidative stress. Therefore, these metabolic abnormalities may be neurochemical correlate of an increased risk to develop alcoholism.Acknowledgements
This research was supported by the Brain Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (PG2016041).References
1. S. Chawla, S. Wang, P. Moore, J. H. Woo, L. Elman, L. F. McCluskey, E. R. Melhem, M. Grossman, H. Poptani, Quantitative proton magnetic resonance spectroscopy detects abnormalities in dorsolateral prefrontal cortex and motor cortex of patients with frontotemporal lobar degeneration, J Neurol 2010;257(1): 114-121.
2. F Bernardin, A Maheut-Bosser, F Palle et al., Congnitive impairments in alcohol-dependent subjects. Frontiers in Psychiatry 2014;78:1-6.
3. Molina V, Sanchez J, Sanz J, et al., Dorsolateral prefrontal N-acetyl-aspartate concentration in male patients with chronic schizophrenia and with with chronic bipolar disorder. European Psychiatry 2007;22:505-512.
4. Lee E, Jang DP, Kim JJ, et al., Alteration of brain metabolites in young alcoholics without structural changes. Neuroreport 2007; 18:1511-1163.
5. V. Govindaraju, K. Young, and A. A. Maudsley, Proton NMR chemical shifts and coupling constants for brain metabolites, NMR Biomed 2000; 13: 129-153.
Figures