4272
Longitudinal Cerebrovascular Analysis of the Aging Mouse Brain using Contrast Enhanced-MRA1Biomedical Engineering, SUNY Downstate Medical Center and New York University, New York, NY, United States, 2Radiology, NYU School of Medicine, New York, NY, United States, 3Chemical and Biomolecular Engineering, NYU Tandon School of Engineering, Brooklyn, NY, United States, 4NYU School of Medicine, New York, NY, United States, 5New York University School of Medicine, New York, NY, United States
Synopsis
Here we study normal brain aging in wild type C57BL/6 mice and have detected a decline in cerebrovasculature over the two year aging process
Introduction
Abnormal changes in the cerebrovasculature are known to be associated with numerous diseases, many of which rely on pre-clinical mouse models to improve our means of diagnosis and treatment. Such studies, however, depend on a reliable control, but we have yet to see a longitudinal assessment of cerebrovascular changes in wild type (WT) control mice. We propose using 3D in vivo contrast enhanced-magnetic resonance angiography (CE-MRA) to longitudinally study the cerebrovasculature of aging WT mice over 2 years using Gadolinium(Gd)-bound micelles as a long-lived blood pool agent for steady state enhancement. This study not only provides information about the most commonly used inbred mouse strain as a basis for future cerebrovascular disease models, but may also provide insight into the normal brain aging process.Methods
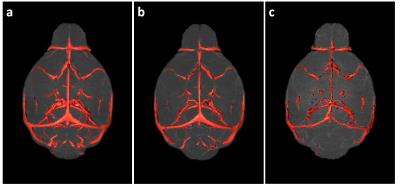
Micelle Preparation: Gd-bound micelles were synthesized via thin-film method1 combining Gd-DTPA, polyethyleneglycol-2000, and Rhodamine-bound lipids. Micelles were sized via dynamic light scattering, their Gd concentration evaluated via inductively coupled plasma optical emission spectrometry (Galbraith Laboratories), and their relaxivity quantified compared to clinical Magnevist (Bruker Minispec, 60MHz). MRA: Studies were performed on female WT C57BL/6 mice aged 2-4months old (mo)-to-26mo (2-4mo N=13, 14-16mo N=5, 24-26mo N=6) with a subset imaged and sacrificed for histology to validate MRA findings. Micelles were administered via femoral injection prior to MRA on a 7T Bruker micro-MRI using a modified 3DGE sequence acquiring respiratory-gating signal to retrospectively correct for motion2 (TE/TR= 4.07/50ms, FA=34°, BW=75KHz, NAV=1, NR=3, Resolution=(100um)3, 87min). MRA Analysis: All angiograms were spatially aligned using Mouse Imaging Centre software3 and tools from the Montreal Neurological Institute. Following alignment, brains and sub-regions were virtually extracted (Amira) (Figure1) and cerebrovasculature was quantified using intensity-based segmentation defining vasculature as the regional mean signal intensity + 2 standard deviations (Image J) (Figure 2). Significance was assessed via one-way ANOVA test and Bonferroni post-test to compare groups. Histology: Immunohistochemistry was performed on formalin-fixed paraffin-embedded brains (N=6). Each slide contained one 5um coronal brain section from each age group for direct comparison. We chromogenically labeled CD31(PECAM-1) for endothelial cell detection and VEGFR1/flt1 as a marker of vascular activation involved in angiogenesis and vessel permeability4 on sequential tissue sections. To count CD31-stained vessels, three independent blinded reviewers counted vessels in 10 high magnification regions in each cortex and hippocampus.Results and Discussion
Results
Micelles: Gd-micelles maintain an average diameter of 15nm+/-2nm with relaxivity values at 60MHz of r1=13.05mM-1s-1+/-0.47 and r2=18.04mM-1s-1+/-0.55, r2/r1=1.38+/-0.009 (N=4), compared to Magnevist r1=3.29mM-1s-1 and r2=3.31mM-1s-1, r2/r1=1.01.
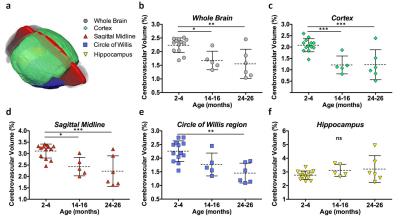
MRI: Quantification of the whole brain showed a significant decrease in detectable cerebrovasculature in the first (p<0.05) and second (p<0.01) year of aging (Figure3b). The cortex (Figure3c) and the sagittal midline (Figure3d), a region with large distinguishable vessels, both showed significant decreases in vasculature after one (cortex: p<0.001, midline: p<0.05) and two (cortex: p<0.001, midline: p<0.001) years. A slab at the base of the brain containing the circle of willis, a vital circulatory anastomosis, showed a significant reduction over two years (p<0.01) (Figure3e). The hippocampus showed no significant changes (Figure3f).
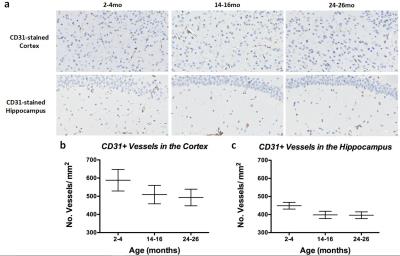
Histology: CD31 staining in the cortex (Figure4a, top panel) and hippocampus (Figure4a, bottom panel) revealed decreased vessel density between 2-4mo and 14-16mo brains in both regions (Figure4b and 4c), with minimal decrease between 14-16mo and 24-26mo brains. VEGFR1-stained hippocampuses of each age group compared to the same anatomic region on the sequential CD31-stained slide (Figure5) revealed that CD31-stained vessels in the 2-4mo hippocampus do not show VEGFR1 staining, while the vessels in the 14-16mo show no-to-moderate VEGFR1 staining and CD31-stained vessels in the 24-26mo brain show high correlation with VEGFR1 staining (Figure 5, left, middle, and right panels, respectively).
Discussion
Using CE-MRA, we detected a cerebrovascular decline over two years of normal aging in WT C57BL/6 mice. This decline was also seen in sub-regions except the hippocampus. CD31-labeling, however, revealed a decline in vessels in the cortex and the hippocampus, validating MRA-based cortical findings, but revealing an undetected decline in the hippocampus likely below the limit of MRA sensitivity. To investigate if this reduction effected the state of vascular endothelial cells, initial studies compared VEGFR1/flt1 and CD31 staining and may suggest that with the reduction in vasculature, the remaining vessels show higher VEGFR1/flt-1 expression possibly in reaction to ischemia-induced angiogenesis5 or due to vessel permeability. Confirmation of this theory requires ongoing immunofluorescence assessment.
Conclusion
This work reports an age-dependent cerebrovascular decline in longitudinally-monitored WT animals using CE-MRA, validated with IHC. These surprising results stress the need to study control strains of transgenic cerebrovascular disease models and provides insight into vasculature changes during brain aging.Acknowledgements
This work was performed at the Preclinical imaging core; a shared resource partially supported by the NYUCI Center Support Grant, “NIH/NCI 5P30CA016087”, the NIBIB Biomedical Technology Resource Center (NIH P41 EB017183) and by NIH grant UL1 TR00038 from the National Center for Advancing Translational Sciences (NCATS).References
1.Briley-Saebo KC, et al. Circulation 2008.117(25):3206-15. 2.Nieman BJ, et al. Magn Reson Med 2008. 61(5):1148-57. 3.Friedel M, et al. Frontiers in Neuroinformatics 2014.8(67):1-21. 4.Shibuya M. Angiogenesis 2006. 9:225-30. 5.Lennmyr F, et al. J Neuropath and Exper Neuro 1998. 57 (9): 874-82.Figures