4271
Aging effects on kurtosis measures of limbic and association white matter tracts1Department of Radiology, Children's Hospital of Philadelphia, Philadelphia, PA, United States, 2Department of Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 3Department of Imaging, Huntington Medical Research Institutes, 4Department of Radiology, University of Texas Southwestern Medical Center, 5Department of Radiology, Johns Hopkins University
Synopsis
Numerous studies have revealed that DTI-derived metrics are sensitive to the microstructural changes of the aging white matter tracts. However, microstructural changes associated with non-Gaussian water diffusion cannot be quantified by DTI-derived metrics, but uniquely quantified by DKI-derived metrics. Little is known on the progressive white matter microstructural changes measured by DKI-derived metric during aging. In this study, we found that the measurements of DKI-derived mean kurtosis (MK) decrease heterogeneously across white matter tracts, characterized with significant MK decreases in limbic tracts including fornix and cingulum and insignificant MK decreases in the association tracts.
Purpose
Numerous studies have revealed that DTI-derived metrics are sensitive to the microstructural changes of the aging white matter tracts [1,2]. However, microstructural changes associated with non-Gaussian water diffusion cannot be quantified by DTI-derived metrics, but uniquely quantified by the metrics [3] derived from diffusion kurtosis imaging (DKI) [4]. In this study, we aimed to accurately reveal the progressive and heterogeneous microstructural changes characterized by DKI at the white matter (WM) skeletons during aging.Methods
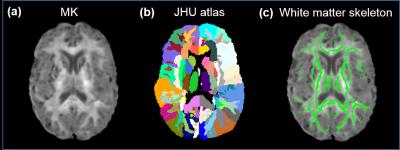
Subjects and data acquisition: 52 healthy subjects with the age of 60 to 82 years were recruited. All scans were performed on a 3T Philips Achieva system (Best, the Netherland). Multi-shell diffusion MR images (dMRI) were acquired using single-shot echo-planar imaging (EPI) and SENSE=2.3 with the following parameters: three b-values: 0, 1000 and 2500 s/mm2; 32 independent diffusion gradient directions; TR/TE=6200/62ms; FOV=224x224mm2; in-plane imaging resolution=2x2mm2, slice thickness=2.2mm with 65 slices without gap covering the entire brain. The multi-shell dMRI data of 51 subjects was used for the following analysis after discarding dMRI of one subject with incomplete acquisition. Fitting of diffusion kurtosis and tensor: Diffusion-weighted images were first corrected for head motion using affine transformation with DTIstudio. The mean kurtosis (MK) was calculated after fitting diffusion kurtosis [4] using the DKE package (http://academicdepartments.musc.edu/cbi/dki/). Fractional anisotropy (FA) maps were obtained after diffusion tensor fitting with DTIstudio. Calculation of MK of a specific tract on white matter skeleton: FA maps of individual subjects were registered to the template in JHU atlas [5] using FSL (http://www.fmrib.ox.ac.uk/fsl). TBSS [6] of FSL was adopted to extract the WM skeleton with the averaged FA map in the template space. The WM skeleton and JHU atlas were inversely transferred to the native space. The MK of a specific WM tract was calculated in the native space using the transferred JHU atlas labels overlaid on the WM skeleton as the region of interests (ROI), as demonstrated in Fig 1. The WM skeleton was used to alleviate partial volume effects. General linear model was employed to investigate the relationship between age and MK values of the limbic and association white matter tracts.Results
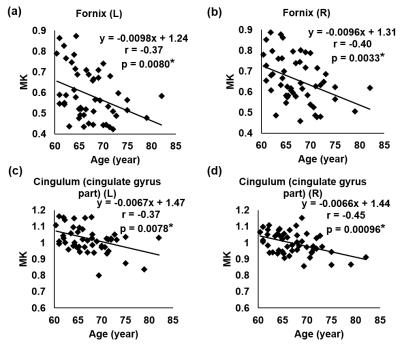
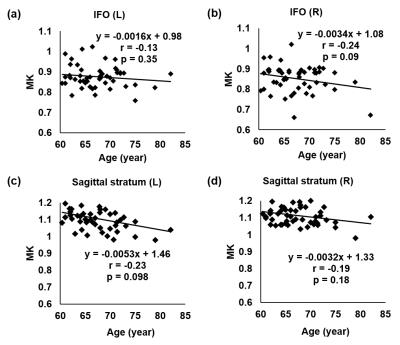
Fig 2a-2d show significant MK decreases (p<0.05) in limbic white matter tracts, namely the left and right fornix and cingulum in the cingulate gyrus, while Fig 3a-3d show insignificant MK decrease (p>0.05) in the left and right inferior fronto-occipital fasciculus (IFO) and sagittal stratum. Within the limbic tracts, the MK values of cingulum are higher than those of fornix. It is clear from Fig 2 and Fig 3 that MK decreases are heterogeneous among different white matter tracts during aging.Discussion and conclusion
Significant MK decreases were found in the limbic white matter tracts, but not in association white matter tracts, indicating heterogeneous aging effects on the microstructure of different white matter tracts. We adopted a method combining DKI measures and WM skeleton to obtain relatively more accurate kurtosis measures of the aging white matter. With microstructural changes based on conventional DTI-derived metrics characterizing Gaussian diffusion well documented in the literature [1,2], microstructural changes based on DKI-derived metrics offer fresh insights on the non-Gaussian diffusion properties in the brain white matter during aging. As limbic tracts play key roles in limbic function, significant MK decreases of limbic tracts may indicate early functional decline, before behavioral or clinical manifestation of mild cognitive impairment (MCI). The underlying neuropathology of MK decreases are not completely known, but are probably associated with decreases of diffusion barriers contributed by myelin and axonal loss. Our findings suggest MK measurement may serve as potential imaging markers to differentiate changes among white matter tracts in aging. The investigation of MK changes in all WM tracts of the aging brain is under way.Acknowledgements
This study is funded by NIH UL1TR001105, MH092535, MH092535-S1 and HD086984. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.References
[1] Wozniak JR, et al. Advances in white matter imaging: a review of in vivo magnetic resonance methodologies and their applicability to the study of development and aging. Neuroscience & Biobehavioral Reviews, 2006; 30(6): 762-774.
[2] Madden DJ, et al. Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochim. Biophys. Acta., 2012; 1822(3): 386-400.
[3] Coutu JP, et al. Non-Gaussian water diffusion in aging white matter. Neurobiology of aging, 2014; 35(6): 1412-1421.
[4] Jensen JH, et al. Diffusional kurtosis imaging: the quantification of non-Gaussian water diffusion by means of Magnetic Resonance Imaging. Magnetic Resonance in Medicine, 2005; 53(6):1432-1440.
[5] Susumu M, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage, 2008; 40(2): 570-582.
[6] Smith SM, et al. Tract-based spatial statistics: voxelwise analysis of multisubject diffusion data. Neuroimage. 2006; 31(4):1487–1505.
Figures